Titration Curve of a Weak Acid
advertisement

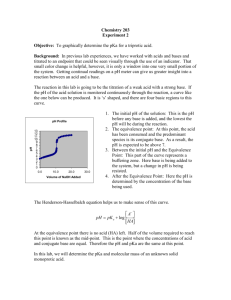
Titration Curve of a Weak Acid Amina Khalifa El-Ashmawy, Ph.D. Collin College Department of Chemistry Introduction: Titration is an analytical process whereby two reactant solutions are carefully reacted in controlled environment. The reactant that is under study is called the analyte while the reactant added to the analyte is called the titrant. Titrations are usually carried out for acid-base neutralization. These can be conducted in two ways: 1) with an indicator, and 2) with a pH meter or probe. The equivalence point is when the reaction solution contains amounts of acid and base consistent with the stoichiometric ratio. The indicator, if carefully chosen, changes color about the same time as the equivalence point is reached. The color change of the indicator is the end point. You have already experimented with indicator titrations. Titration Curves: When a pH meter is used to monitor the reaction, the experimenter records the pH of the solution as a function of total volume of titrant added. The volume of titrant is the independent variable, the one that is controlled. The pH is the dependent variable as it is the one measured as the outcome of manipulating the independent variable. Once the volume of titrant – pH data is plotted, the resulting graph is called a titration curve. The plot generally makes an S-shaped or inverted S-shaped curve depending on whether the titrant is an acid or base. If the analyte is an acid and titrant is base, the titration curve will be an S-shaped curve (figure 1). If the analyte is a base and titrant is acid, the titration curve will be an inverted S-shaped curve (figure 2). Figure 1. Titration curve of a acid with base Copyright© 2011 Amina Khalifa El-Ashmawy Figure 2. Titration curve of a base with acid 14 12 Ka = 1.0X10-10 10 Ka = 1.0X10-8 Series1 8 Series2 pH Ka = 1.0X10-6 Series3 6 Series4 Ka = 1.0X10-4 Series5 Series6 4 Ka = 1.0X10-2 2 Strong Acid 0 0 10 20 30 40 50 60 70 Volumeofof1.00 1.00 NaOH Volume MM of NaOH Figure 3. Titration curves of different monoprotic weak acids with strong base. The slope of the flat regions on the left and right tells you something about the acid or base. The flatter the region the stronger the substance (as in the figure above); the more incline there is in a region the weaker the substance. Once a little bit of titrant, whether it is a strong acid or strong base, has been added to the weak substance, the conjugate species is generated, which results in a buffer solution. The point of inflection in the curve is the equivalence point. The pH at the equivalence point depends on the chemical species that remain in solution. For example, in the titration of HCl (strong acid) with NaOH (strong base), the species in solution at the equivalence point are Na+ and Cl- in H2O. Let’s explore what might happen if you think of H2O as H+OH-. Copyright© 2011 Amina Khalifa El-Ashmawy The reaction of ions with water is called hydrolysis. The Na+ might combine with OH- from the water while Cl- might combine with H+ to give: Na+(aq) + H2O(l) NaOH(aq) + H+(aq) and Cl-(aq) + H2O(l) HCl(aq) + OH-(aq). Looking closely at what we are suggesting for the products, assuming the reactions occur, we see that we are forming NaOH (a strong base) and HCl (a strong acid). By definition, strong species completely ionize or dissociate in solution and will not come together as written in the above equations. Hence, there is no increase in either the H+ or OH- in solution. The solution pH at the equivalence point will be 7.000. Na+(aq) + H2O(l) No Reaction and Cl-(aq) + H2O(l) No Reaction. Now, let’s take a look at what happens at the equivalence point of a weak acid with strong base. In the case of titrating formic acid (HCOOH) with NaOH, the pH at the equivalence point will depend on the ions present, which are Na+ and HCOO-. We can write equations for the hydrolysis by these ions as we did before. The equations would be Na+(aq) + H2O(l) No Reaction and HCOO-(aq) + H2O(l) HCOOH(aq) + OH-(aq). In the second equation we are forming a weak acid, which will exist in its molecular form in solution. This will result in equilibrium, and we see that there is increased OH- concentration in solution. Hence, the pH at the equivalence point of a weak acid and a strong base will be higher than 7.000 due to hydrolysis of the conjugate base. - HCOO HCOO (aq) (aq) ++HH 2O(l) 2O(l) -HCOOH HCOOH(aq) OH(aq) (aq). (aq) + +OH The pH of such a solution is dictated by the Kb of the conjugate base HCOO-. Its value depends on the Ka of the weak acid HCOOH and can be calculated by Kb = Kw/Ka. Polyprotic Acids: Polyprotic acids ionize one step at a time. For a weak diprotic acid, the equilibria reactions are: HCOO HCOOH H2A(aq) HA-(aq)(aq) + H+3O+OH . (aq) (1) (aq) ++HH 2O(l) 2O(l) Copyright© 2011 Amina Khalifa El-Ashmawy HCOO HCOOH HA-(aq) O(l) A2-(aq) +(aq)H3+O+OH . (aq) (2) (aq) ++H2H 2O(l) The neutralization of such acids also occurs in steps where the first proton is neutralized, then the second proton is neutralized. H2A(aq) + NaOH(aq) NaHA(aq) + H2O. (1) NaHA(aq) + NaOH(aq) Na2HA(aq) + H2O. (2) Each reaction will have its own, distinct equivalence point. The pH at each equivalence point depends on the respective Ka value. The titration curve looks like two S-shaped curves connected to each other. Figure 4. Titration curve of a diprotic acid with strong base Features of the Titration Curve: The equivalence point is an important feature of a titration curve because it allows us to determine the concentration of an unknown acid or base. At the equivalence point, the reactants are in stoichiometric amounts such that the solution contains [H+] = [OH-] prior to any hydrolysis that may occur. If you titrate a monoprotic acid of unknown concentration, you can determine its concentration stoichiometrically from the amount of base needed to reach the equivalence point. For example, you want to titrate a 20.00 mL aliquot (liquid sample to be analyzed) of unknown monoprotic acid. If it takes 18.78 mL of 0.10 M KOH to reach the equivalence point in the titration of a 20.00 mL aliquot of the unknown monoprotic acid, you can determine that Mol KOH = (18.78 mL)(1 L/1000 mL)(0.10 mol/L) = 0.001878 mol KOH. Note: we will not round any numbers in the calculations to avoid rounding errors. Since the reaction is: HA(aq) + KOH(aq) KA(aq) + H2O(l), there is a 1 to 1 molar ratio of KOH to HA. We then know that the 20.00 mL aliquot of acid contained 0.001878 mol HA. Therefore, the concentration of the acid is Copyright© 2011 Amina Khalifa El-Ashmawy (0.001878 mol HA)/[(20.00 mL)(1 L/1000 mL)] = 0.094 M HA. Another important feature is the point of half-neutralization. With the concentration of the unknown weak acid determined, let’s calculate what is present in solution at half-neutralization. You have: (0.02000 L HA)(0.094 mol/L) = 0.001878 mol HA. At half-neutralization, we know that moles KOH = ½ moles HA = 0.000939 moles. From the moles of KOH and the known molarity of KOH, we can calculate the volume of KOH required for half-neutralization. (0.000939 mol KOH)/(0.10mol/L) = 0.00939 L HA(aq) + KOH(aq) KA(aq) + H2O(l) Initial Moles: 0.001878 0.000939 Change: -0.000939 -0.000939 Final Moles: 0.000939 0.00 0.00 0.00 +0.000939 +0.000939 0.000939 0.000939 From the reaction chart above, we see that KOH is the limiting reactant and will completely react. There remains (0.001878 – 0.000939) mol HA, or 0.000939 mol HA. The other 0.000939 mol HA already reacted to produce KA, which contains the common ion A -. In solution at halfneutralization, then, you have [HA] = (0.000939 mol)/(0.02939 L) = 0.0319 M and [A-] = (0.000939 mol)/(0.02939 L) = 0.0319 M. The [H3O+], hence the pH of the solution, can be determined by setting up the RICE (Reaction, Initial concentration, Change, Equilibrium concentration) table and the Ka expression for HA. HA + 0.0319 -X 0.0319 –X H2 O A- + 0.0319 +X 0.0319+X H 3 O+ 0 +X X For weak acids we can approximate [HA] ~ 0.0319 M and [A-] ~ 0.0319 M. Plugging into the Ka expression we get (X) (0.0319) [H3O+] [A-] Ka = = = X = [H3O+] (0.0319) [HA] Copyright© 2011 Amina Khalifa El-Ashmawy So, we see that at half-neutralization [H3O+] = Ka and pH = pKa. The Problem: You will be given an unknown weak polyprotic acid to titrate with 0.10 M NaOH using a pH probe. From the titration curve you will determine the Ka values for each proton and the unknown acid concentration. Possible unknowns are malonic, succinic, phosphoric, glutaric or adipic acids. Questions to answer prior to performing the lab: Following are some questions to consider before starting the lab. What is a diprotic acid? Triprotic acid? Write the ionization equations for the protons of a triprotic acid. Do you need to use an indicator when you are doing a pH titration? What is the pH of 0.10 M HCl? 0.10 M CH3CO2H? 0.10 M H2SO4? How many trials of each titration should you carry out in order to obtain dependable results? Critical Data/Discussion to Include in Your Lab Report: Data table Titration curve for each titration trial Calculation of acid concentration Calculation of both Ka values Discussion of why the second Ka is smaller than the first Ka value (removing a proton from an anionic species as opposed to removing a proton from an electrically neutral species.) Discussion of the possible identity of the unknown acid based on the Ka values calculated Work cited Copyright© 2011 Amina Khalifa El-Ashmawy