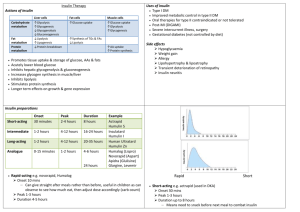
gENEriC DrUgS
advertisement

Toxi Drug Info September 2009 Event Update Volume 3 Issue 3 2 Generic Drugs Policy Watch out 3 Medication Safety Alerts Toxi-Review 7 Organophosphorous Extemporaneous 8 Tacrolimus In Oral Suspension Question of the month 9 Oral Hypoglycemic Agents During Pregnancy Pharmacovigilance 11 Minimizing Adverse Drug Events In Elderly Review 13 Electronic Prescribing, Benefits Versus Risks Medication Safety 15 Insulin Glargine & Cancer Toxicology Quiz 16 Rumors vs Truth 17 HAAD Formulary Update 18 News Update 19 Safety information on Tamiflu® & Relenza® for influenza A H1N1 األدويـة و المنتجـات الطبيـة/إدارة الصيدلة p d i c . h a a d . a e Pharma/Medicines & Medical Products Department NEWS UPDATE Health Authority - Abu Dhabi, Pharma/Medicines & Medical Products Department (PHM) Chief editor Dr. Abdullah Hassan, Manager, PHM Executive Editors Dr. Mohammed Abuelkhair, Head, Drugs & Medical Products Regulation Section, PHM Dr. Yasser Sharif, Head, Medications & Medical Products Safety Section, PHM Contributing Authors Dr. Shajahan Abdu, Pharmacovigilance Officer (Generic Drugs: From Policy To Implementation) Dr. Kefah Al Qawasme, PDIC Officer (Medication Safety Alerts) Dr. Yasser Sharif, Head, Medication & Medical Products Safety Section & Dr. Sherif El Ghandour, PDIC Officer (Organophosphorous Poisoning Review) Dr. Sherif El Ghandour, PDIC Officer (Is The Use Of Oral Hypoglycemic Agents Contraindicated During Pregnancy?) Dr. Maysoon Saffarini, PDIC Officer (Tacrolimus In Oral Suspension Form) Dr. Sahar Fahmy, Pharmacovigilance Officer (Minimizing Adverse Drug Events in Elderly) Dr. Majdoline Abu Musallem, Import and Export Regulation Officer (Electronic Prescribing, Benefits Versus Risks) Dr. Hadaya Gharibyar, PDIC Officer (Insulin Glargine (LANTUS®) and Cancer Association: A Closer Look at the Literature) Dr. Rania Al Dwiek, PDIC Officer (Rumors Versus Truth) Acknowledgment We thank Dr. Kefah Al Qawasme for actively participating in producing this issue. We value your comments. Please provide us with your feedback regarding the contents by reaching us at: pdic.haad.ae Poison & Drug Information Center Mission l l To enhance the level of pharmaceutical care through enabling Physicians, Pharmacists, Nurses and other members of the healthcare team, to access accurate and relevant poison and drug information. To protect the health of community by providing immediate, uninterrupted, high quality emergency telephone advice for poison exposures and to serve as the primary resource for poison education, prevention and treatment in Abu Dhabi. Vision l l l Serve our community and healthcare professionals by providing health information and medical expertise regarding poisonings, drug information and adverse drug events. Conduct research on the prevention and treatment of toxicity. Provide accessible, reliable and responsive services through state-of-the-art information technology systems. Values Excellence, Integrity, Innovation, Collaboration- and Leadership. We embody these qualities in our commitment to enable accessible healthcare for all. The PDIC serves all UAE healthcare facilities and its medical and healthcare staff. It also serves and receives inquiries from the general public nationwide Poison and Drug Information Center Services 1. Poison management and prevention l PDIC will assist the healthcare professionals and general public with poison emergencies by providing quick and accurate information in regard to the following: l Signs and symptoms of toxic exposures to specific poisons l Overdoses with medications l Exposure to household chemicals, cosmetics,.etc. l Herbals, traditional medicines and dietary supplements l Industrial chemicals and exposure l Bites and stings from venomous creatures l Toxic plants l l l l First aid advice on management of toxic exposure Advice on detailed medical management of poisonings Antidotes uses and availability Poison prevention 2.Safe Use of Medications PDIC plays an important role in promoting medication safety through l Dissemination of information on the latest recommendations regarding safe medication use to all health care professionals l Tracing and follow up medication errors, adverse drug events and side effects, circulates the relevant findings and recommendations to all medical and health care staff. l Follow up on adverse effects of herbal and health supplements 3.Educational and Services Program PDIC is an educational and service center designed to support poison and drug information provided to the public and health care professionals in regard to the following areas: l Education of the public in the areas of poisoning prevention and first aid. l Education of health care professionals in the areas of clinical toxicology, poisoning epidemiology, poisoning prevention, toxicological diagnosis and care. l Provision of drug information to practitioners, patients and their families and other personnel (students, faculty teachers, universities) to optimize medication use and achieve specific health outcomes. 4.HAAD Medications Formulary PDIC continuously strives for improved patient safety, appropriate use of medications and improved access to pharmaceuticals through its active participation in Formulary Committee. PDIC supports the Formulary Committee through: l Evaluating new medication l Developing therapeutic guidelines l Updating professionals on new labeling of established products 5. Coordination of Drug Research and Promoting Research on Medication Use The center supports in the review of pharmaceutical clinical research protocol, as an active participant in the investigation review board. As part of its responsibility, the center keeps track of pharmaceutical clinical research at health care facilities by coordinating and conducting research regarding: l Drug pricing, affordability, accessibility and availability of medication l Analysis of pharmacoeconomic studies and their relevance to the community l Situational analysis of drug use problems. l Prescribing patterns EVENT UPDATE GENERIC DRUGS: FROM POLICY TO IMPLEMENTATION Introduction G eneric Drugs have existed throughout the history of the pharmaceutical industry, but modern generic firms emerged only in the mid 1960s in the context of the shakeup of regulatory arrangements in USA following the thalidomide tragedy. The 1984 US Drug Price Competition and Patent Restoration (Hatch-Waxman) Act was the decisive moment in the development of the generics industry in the world’s major pharmaceutical market. The Hatch-Waxman Act provided for facilitated market entry for generic versions of all post-1962 approved products, in exchange for an extension of the patent period (Congressional Budget Office 1998). This opened the floodgates for generic competition of pharmaceutical products, creating the modern generic pharmaceutical industry. However, it took until the 1990s for generic competition to alter significantly the dynamics of the US pharmaceutical market. A key driver was the spearheading by Health Maintenance Organizations and Pharmaceutical Benefit Management companies of cost containment measures such as generic prescribing, brand substitution by pharmacists, and reimbursement on the basis of the cheapest brand. By 1997, 63% of Health Maintenance Organizations are said to have imposed mandatory generic substitution.1 The Waxman-Hatch Act is estimated by the Congressional Budget Office to have saved consumers US$ 8-10 billion in 1994, and presumably at least as much in subsequent years.2 The World Health Organization and pharmaceutical experts have recommended that Generic Drug Policies be implemented to improve the availability of affordable medicines, as it is one of the most visible indicators of quality health services.3 However, policy in this area is fraught with contention that arises inescapably from the fact that generic drugs invite cost effective comparisons and make substitution possible. On one hand, generic competition is seen as a cost containment, and as also a way of adding to innovative pressures on originator firms. The opposing perspective is to consider the growth of Generics as opposing to the growth of research based industry and Generic substitution and related measures as an intrusion on the professional authority of doctors. HAAD Initiatives Health Authority Abu Dhabi (HAAD) introduced ‘Unified Prescription Form’ and mandated Generic prescribing in March 20094, as part of the ongoing efforts to improve the prescribing practices in the Emirate of Abu Dhabi. The comprehensive generic drugs policy, published in August 2009, provided for additional strengthening of controls over the generic prescribing and dispensing practices, ensuring that patients have access to affordable and quality medicines appropriate to their clinical needs. HAAD Pharma / Medicines and Medical Products Department also organized 4 workshops in August 2009 to educate the stakeholders on the new Generic Drug Policy. The workshops were held at HAAD auditorium and specifically targeted pharmacists, physicians & allied health care professionals, insurance companies, pharma industries and drug agents. Generic Drugs Policy5 HAAD Generic Drug Policy is well balanced and applies to pharmacists, all other healthcare professionals who have the authority to prescribe drugs (physicians), and healthcare providers in whose facilities such pharmacists or physicians are employed. Key features: Duties on Physicians Physicians must prescribe the medication for a patient in the standard prescription format (developed by HAAD) and must refer to the medication in its Generic Name (active Pharmaceutical ingredient) unless one of the exceptions set out below applies. Exceptions: 1. A physician who prescribes a medication for a patient may choose not to prescribe the medication by reference to its Generic Name where that medication is: (a)a drug registered with the Federal Ministry of Health as an ‘over-the-counter’ drug or ‘general sale product’, (b)a dietary supplement or food supplement, (c)a herbal or homeopathic remedy, (d)a vaccine or insulin preparation, or (e)Narrow Therapeutic Range Drugs (Refer Appendix B of HAAD Generic Drugs Policy) 2. A physician who prescribes a medication for a patient may also choose not to prescribe the medication by reference to its Generic Name, where to do so would place that patient’s health at risk because the patient: (a)has a documented history of an allergic or other adverse reaction to a particular Generic Drug, or (b)has previously suffered unsuccessful therapeutic control with a Generic Drug. In such an instance, the physicians must l also write the phrase ‘Do Not Substitute’ or the initials ‘DNS’ next to item prescribed, l document the reasons for prescribing a brand-name drug in the patient record, and l be able to give the reasons for prescribing a brand-name drug, if requested to do so by HAAD. Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 I 2 Duties on Pharmacists 1. The pharmacist must review a patient’s medication on each occasion before dispensing it to the patient. 2. May usually (subject to certain exceptions) substitute appropriate generic drugs for brand-name drugs. 3. Must not dispense a generic drug in place of a prescribed brand-name drug where the prescribing physician has placed the phrase ‘Do Not Substitute’ or the initials ‘DNS’ next to the name of the item being prescribed. 4. Must not engage in the therapeutic substitution of drugs (e.g. replace ranitidine for cimetidine or ofloxacin for ciprofloxacin, etc.) 5. In dispensing to patients on long term therapy: (a)be consistent over time in the selection of generic drugs that are dispensed, and (b)where it is necessary to dispense a Generic Drug under a different brand name to that of the same generic drug which was previously dispensed to the patient, explain to the patient that the medication remains the same in substance. The Duty on Healthcare Providers Each healthcare provider must ensure that healthcare professionals employed by it at their healthcare facilities comply with any duties placed on them under this Policy. Request for Exemption from Generic Prescribing Physicians may submit a request to HAAD, if they consider that a particular drug/drug molecule (other than those exempted by HAAD), needs to be permanently exempted from generic prescribing. All requests in this regard should be made only through the ‘Exemption Request Form’6, and submitted directly to HAAD Pharma/Medicines and Medical Products Department or through email or fax (see contact details below). A committee comprised of experts from ‘Pharma/Medicines and Medical Products Department’ and ‘Pharmacy and Therapeutic Committee’ at HAAD will scientifically review the physician’s request for exemption. If the committee recommends the drug for exemption from generic prescribing, it will be communicated to all healthcare professionals through HAAD health advisory and /or circular. For more information on generic prescribing or Generic Drugs Policy, please contact: HAAD Pharma/Medicines and Medical Products Department Pharmacovigilance Center Phone: 02 4193 348, 580 586. Fax: 02 4496679, Email: pv@haad.ae References: 1. Mrazek M., Mossialos E. Increasing demand while decreasing costs of generic medicines, The Lancet 2000;356(9244):1784-85 2. Congressional Budget Office 1998, p. ix. 3. Bulletin of the World Health Organization | January 2005, 83 (1) 4. HAAD CEO circular no (03/09)to all public and private sector hospitals. 5. HAAD Generics Drug Policy PHP/PHM/P0003/09, Date of Approval: August, 2009. Also available at http://www.haad.ae/haad/tabid/82/Default.aspx. 6. HAAD Generic Drugs Policy, PHP/PHM/P0003/09, Appendix C. MEDICATION SAFETY ALERTS Safety information on oseltamivir (Tamiflu®) and zanamivir (Relenza®) for pandemic swine influenza A H1N1 D uring August 2009, the Medicines and Health Care products Regulatory Agency (MHRA) at the UK posted on their website the information below concerning the safety of oseltamivir and zanamivir. Tamiflu and Relenza are both neuraminidase enzyme inhibitors. They act by inhibiting entry of influenza virus into uninfected cells and preventing the release of recently formed virus particles from infected cells. Tamiflu is given orally (capsules and solution) and Relenza is given by inhalation (Diskhaler system). Both have substantial experience of use and favorable benefit-risk profiles. Tamiflu® Side effect profile The most common side effects of Tamiflu are nausea, vomiting, diarrhoea, abdominal pain, and headache. These may usually occur after the first dose and will usually stop as treatment continues. The frequency of these effects is reduced if Tamiflu is taken with food. More serious side effects are very rare. 3 i Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 The product information for Tamiflu lists neuropsychiatric disorders (reports of convulsions and delirium) in the side-effects section. These events were added to the product information as a precautionary measure. A causal association between Tamiflu and the reported events has not been established. Drug interactions Clinically important drug interactions with Tamiflu are unlikely, including those involving competition for renal tubular secretion. However, care should be taken when prescribing Tamiflu for patients who are taking co-excreted medicines with a narrow therapeutic margin (eg, chlorpropamide or methotrexate). No dose adjustment is required when coadministering with probenecid in patients with normal renal function. Coadministration of probenecid, a potent inhibitor of the anionic pathway of renal tubular secretion, results in an approximate two-fold increase in exposure to the active metabolite of oseltamivir. Renal impairment Dose adjustment is recommended for adults with severe renal insufficiency (creatinine clearance ≤ 30 mL/min). Tamiflu is not recommended for patients with a creatinine clearance of ≤ 10 mL/min or in those undergoing dialysis. Watch Out Relenza® Relenza is delivered by inhalation using a Diskhaler. It has been recommended for anyone with a kidney disorder or who may be pregnant. Reference: 1. UK Medicines and Health Care products Regulatory Agency (MHRA). On line through: http://www.mhra.gov.uk/Publications/Safetyguidance/DrugSafetyUpdate/ CON054505. Accessed date: August 16th, 2009 Side effects Tumor Necrosis Factor (TNF) Recognized side effects to Relenza are very rare, but may include allergic-type reactions such as swelling of the face, mouth, or throat; skin rash; or hives. Acute bronchospasm or serious decline in respiratory function (or both) have been seen in patients with a history of asthma or chronic obstructive pulmonary disease (COPD), and in those without a history of respiratory disease (see below). Blockers (marketed as Remicade®, Enbrel® and Humira®) and Cancer The product information for Relenza also lists neuropsychiatric disorders as a possible side effect of the medicine. As with Tamiflu, these events were added to the product information as a precautionary measure and a causal association with Relenza is uncertain. Patients with asthma or COPD Patients with severe asthma should not receive Relenza unless close medical monitoring and appropriate clinical facilities are available to prevent bronchoconstriction. In patients with persistent asthma or severe COPD, management of the underlying disease should be optimised during Relenza treatment. How To Report Suspected Adverse Reactions From Tamiflu® and Relenza® Please report all suspected ADRs to Tamiflu and Relenza to HAAD Pharma /Medicines and Medical Products Department/ Pharmacovigilance Center by any of the following: Phone: 02 4193 348, 580 586. Fax: 02 4496679. Email: pv@haad.ae l Please remember to include the following important information in your report: - Patient age - Indication (prophylaxis or treatment) - Outcome of the ADR - Information on any underlying risk factors for the ADR or for influenza complications. Also mention if there are no known risk factors - Any other information about the patient or additional clinical details that will help us in our assessment of the case l When influenza A H1N1 vaccines become available, the Pharmacovigilance Center should also be contacted to report suspected ADRs to these vaccines Before this influenza A H1N1 outbreak, use of these medicines in the UAE and worldwide was limited. It is possible that the wider prescribing of these medicines in a pandemic situation may reveal rare effects that have not previously been reported. Therefore, it is important that suspected ADRs to Tamiflu and Relenza are reported to us. If you suspect that a patient has experienced an adverse reaction to these antivirals, please report as outlined above. l O n August 4th 2009, US Food & Drug Administration (FDA) notified healthcare professionals that it has completed its assessment of tumor necrosis factor (TNF) blockers and has concluded that there is an increased risk of lymphoma and other cancers associated with the use of these drugs in children and adolescents. This new safety information is now being added to the Boxed Warning for these products. FDA has also identified new safety information related to the occurrence of leukemia and new-onset psoriasis in patients treated with TNF blockers. The current prescribing information for TNF blockers does contain a warning for malignancies, but does not specifically mention leukemia. FDA is also requiring updates to the current Medication Guide to help patients understand the risks associated with TNF blocker therapy. TNF blockers are approved for the treatment of one or more of a number of immune system diseases including juvenile idiopathic arthritis (JIA), rheumatoid arthritis, psoriatic arthritis, plaque psoriasis, Crohn’s disease, and ankylosing spondylitis. Recommendations for Healthcare Professionals: Discuss with patients and families the increased risk of developing cancer in children and adolescents, taking into account the clinical utility of TNF blockers, the risks/benefits of other immunosuppressive therapies, and the risks associated with untreated illness. l Be aware of the possibility and monitor for the emergence of malignancies during and after treatment with TNF blockers. l Be aware of the possibility and monitor for the emergence or worsening of psoriasis during treatment with TNF blockers, particularly pustular and palmoplantar forms of psoriasis. l Understand that some immune-related diseases, such as Crohn’s, have been shown to increase cancer risk independent of treatment with TNF blockers while for others, such as juvenile idiopathic arthritis (JIA), it is unknown whether there is an increased cancer risk. l Inform patients, their families, and caregivers of the signs and symptoms of malignancies or psoriasis so they are aware of and able to notify their healthcare professional of any unusual signs or symptoms. l Reference: 1. The US Food and drug Administration MEDWATCH. Safety Information and Adverse Event Reporting Program. On line through: http://www.fda.gov/Safety/ MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm175843. htm. Accessed date: August 16th, 2009. Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 I 4 Onabotulinumtoxin A (marketed as Botox®/Botox Cosmetic®), Abobotulinumtoxin A (marketed as Dysport®) O n July 31, 2009, US Food & Drug Administration (FDA) approved the following revisions to the prescribing information of Botox/Botox Cosmetic based on a safety evaluation of the botulinum toxin products: l A Boxed Warning highlighting the possibility of experiencing potentially life-threatening distant spread of toxin effect from the injection site after local injection. l Changes to the established drug names to reinforce individual potencies and prevent medication errors. The potency units are specific to each botulinum toxin product, and the doses or units of biological activity cannot be compared or converted from one product to any other botulinum toxin product. The new established names reinforce these differences and the lack of interchangeability among products. The other botulinum toxin product in this class, AbobotulinumtoxinA (marketed as Dysport), was approved on April 29, 2009 and included the Boxed Warning, REMS, and new established name at the time of approval in the US. Table 1 lists the established name changes and the approved indications for each product. Recommendations for Healthcare Professionals: l Be aware that a Boxed Warning has been added to the prescribing information to highlight that botulinum toxin may spread from the area of injection to produce symptoms consistent with botulism. Symptoms such as unexpected loss of strength or muscle weakness, hoarseness or trouble talking (dysphonia), trouble saying words clearly (dysarthria), loss of bladder control, trouble breathing, trouble swallowing, double vision, blurred vision and drooping eyelids may occur. Understand that swallowing and breathing difficulties can be life-threatening and there have been reports of deaths related to the effects of spread of botulinum toxin. l Be aware that children treated for spasticity are at greatest risk for these symptoms, but symptoms can also occur in adults treated for spasticity and other conditions. l Be aware that cases of toxin spread have occurred at botulinum toxin doses comparable to those used to treat cervical dystonia and at lower doses. l Be aware that no definitive serious adverse event reports of distant spread of toxin effect have been associated with dermatologic use of Botox/Botox Cosmetic at approved doses. l Be aware that no definitive serious adverse event reports of distant spread of toxin effect have been associated with Botox for blepharospasm or for strabismus at approved doses. l Understand that the established drug names of the botulinum products have been changed to emphasize the differing dose to potency ratios of these products. l Understand that botulinum toxin products are not interchangeable. l Be aware that all botulinum toxin products have a Medication Guide and urge patients, their families, and caregivers to review it carefully. l Understand that clinical doses expressed in units are not comparable from one botulinum toxin product to the next. Units of one product cannot be converted into units of another product. l Reference: US Food and drug Administration MEDWATCH. Safety Information and Adverse Event Reporting Program. On line through: http://www.fda.gov/Safety/MedWatch/ SafetyInformation SafetyAlertsforHumanMedicalProducts/ucm164255.htm. Accessed date: August 16th, 2009 Table 1. Summary of FDA-Approved Botulinum Toxin Products Trade Name*NEW Drug NameOLD Drug NameIndication Botox OnabotulinumtoxinA Botulinum toxin type A Botox Cosmetic OnabotulinumtoxinA Botulinum toxin type A Dysport AbobotulinumtoxinA Botulinum toxin type A Cervical dystonia, Severe primary axillary hyperhidrosis, Strabismus, Blepharospasm Temporary improvement in the appearance of moderate to severe glabellar lines Cervical dystonia, Temporary improvement in the appearance to moderate to severe glabellar lines Myobloc** Cervical dystonia RimabotulinumtoxinB Botulinum toxin type B * The marketed trade names and the product formulations have not changed. ** Not available in the UAE and not approved by the Ministry of Health. 5 i Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 Watch Out Mycophenolate mofetil and pure red cell aplasia O n June 2nd, 2009, ROCHE pharmaceutical issued a Dear Healthcare Professional Letter on the association of Mycophenolate mofetil (CellCept) with pure red cell aplasia. The information has been reviewed and endorsed by the European Medicines Agency’s (EMEA) Committee for Medicinal Products for Human Use (CHMP). This letter is posted on the UK Medicines and Health Care products Regulatory Agency (MHRA) and the United States FDA official websites.Mycophenolate mofetil (CellCept) is an immunosuppressant indicated in combination with ciclosporin and corticosteroids for prophylaxis of acute transplant rejection in adults receiving allogeneic renal, cardiac or hepatic transplants, and in children and adolescents (age 2–18 years) receiving renal transplants. Up to April 2009, 41 cases of pure red cell aplasia had been reported worldwide in association with mycophenolate mofetil. Pure red cell aplasia is a type of anaemia in which there is a selective reduction of red blood cell precursors on bone-marrow examination. Some patients were also receiving other medicines that could have contributed to the development of pure red cell aplasia (eg, alemtuzumab, tacrolimus, azathioprine, and co-trimoxazole). In four cases dose reduction, and in 12 cases discontinuation, of mycophenolate mofetil led to resolution of the condition. The mechanism by which mycophenolate mofetil may cause pure red cell aplasia is unknown. Recommendations for Healthcare Professionals: l Dose reduction or discontinuation of mycophenolate mofetil should be considered in patients who develop pure red cell aplasia. Changes to treatment should be done only under specialist supervision to minimize the risk of graft rejection 2. US Food and drug Administration MEDWATCH. Safety Information and Adverse Event Reporting Program. On line through: http://www.fda.gov/Safety/MedWatch/ SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm177397.htm. Accessed date: August 16th, 2009 Leukotriene Inhibitors: Montelukast O n August 28th, 2009, US Food & Drug Administration (FDA) provided healthcare professionals with updated information on the original March 2008 early communication and January 2009 follow-up communication about the ongoing safety review for the leukotriene inhibitors, montelukast. Neuropsychiatric events have been reported in some patients taking montelukast (Singulair). FDA has requested that manufacturers include a precaution in the drug prescribing information (drug labeling). The reported neuropsychiatric events include postmarket cases of agitation, aggression, anxiousness, dream abnormalities and hallucinations, depression, insomnia, irritability, restlessness, suicidal thinking and behavior (including suicide), and tremor. Recommendations for Healthcare Professionals: l l l Patients and healthcare professionals should be aware of the potential for neuropsychiatric events with these medications. Patients should talk with their healthcare providers if these events occur. Healthcare professionals should consider discontinuing these medications if patients develop neuropsychiatric symptoms. References: Reference: 1. UK Medicines and Health Care products Regulatory Agency (MHRA). On line through http://www.mhra.gov.uk/Publications/Safetyguidance/DrugSafetyUpdate/ CON051770. Accessed date: August 16th, 2009 1. FDA MedWatch. On line through: http://www.fda.gov/Drugs/DrugSafety/ PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm165489.htm. Accessed date: August 28th, 2009 Prescription Only Medications (POM) Stop Dispensing without a prescription Dispensing (POM) without A prescription is illegal UAE Federal Law No. 4; Article 11 for the year of 1983 about pharmaceutical Professions and Institutions “The licensed pharmacist should not give any medicine or medicinal preparation without a medical prescription in a clear hand-writing carrying the name of the licensed doctor who issued it, its stamp and date issued.” Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 I 6 eview R I X O T Organophosphorous Poisoning Review Introduction: Organophosphorous compounds (OPs), generally known as cholinesterase inhibitors are widely used pesticides that may cause poisoning after occupational, accidental or suicidal exposure. Poisoning with OPs frequently causes ill-health that may be severe enough to cause death, particularly in developing countries. The number of intoxication cases with OPs is estimated to be 3 millions each year. Fatality rates ranges from 15-20% and according to the WHO around 200,000 people die each year from OPs pesticide poisoning.1 Mechanism of toxicity: OPs primarily inhibit acetylcholinestrase enzyme (AChE) which is found in synaptic junctions and red blood cells, it also inhibits butyrylcholinestrase (psudocholinestrase) which is found in the plasma. Inhibition of AChE leads to accumulation of acetyl choline (Ach) at muscarinic receptors, nicotinc receptors and central nervous system, thereby, disturbing transmission at parasympathetic nerve ending, sympathetic nerve endings, sympathetic ganglia, neuromuscular end- plates and certain central nervous system regions.2 Permanent inhibition of AChE may occur through covalent binding by the OP to the enzyme. This is known as “aging,” and its rate of development is variable and depends on the specific OP. Exposure: OPs are absorbed by the following routes; l Inhalation l Ingestion l Skin absorption Clinical presentation: Signs and symptoms of acute organophosphate poisoning may occur almost immediately or be delayed several hours, depending on the above factors. Clinical manifestations may be classified into: l Muscarinic manifestations include; vomiting, diarrhea, abdominal cramping, bronchospasm, miosis, bradycardia, and excessive salivation and sweating. Fluid losses can lead to systemic hypovolemia, resulting in shock. l Nicotinic effects include muscle fasciculations, tremor, and weakness. Respiratory muscle weakness complicated by bronchorrhea may lead to respiratory arrest and death. l Central nervous system (CNS) poisoning may cause agitation, seizures, and coma. Furthermore, delayed, often permanent peripheral neuropathy may occur. l Two days after recovery from acute cholinergic crisis, some patients may develop proximal muscle weakness that start as neck weakness, progressing to proximal limb weakness and cranial nerve palsies. Respiratory muscle weakness and respiratory arrest may occur abruptly. This is known as “the intermediate syndrome” which is thought to be caused by a redistribution of lipophilic pesticides or result from inadequate oxime therapy. Symptoms may last for 2-4 weeks, and do not usually respond to additional treatment with oximes or atropine.4 7 i Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 In addition, chemical pneumonitis may occur, if a product containing a hydrocarbon solvent is aspirated into the lungs.2 Diagnosis: Diagnosis is made on the basis of clinical suspicion, the characteristic clinical signs, smell of pesticides or solvents, and reduced psudocholinestrase or AChE activity in the blood. However the latter diagnostic assays are not readily available in time to affect the clinical decision. Patients with severe OP poisoning typically present with pinpoint pupils, excessive sweating, reduced consciousness, and poor respiration. The major differential diagnosis is carbamate poisoning, which is clinically indistinguishable, however carbamates do not age AChE, and therefore toxicity is usually brief and self limiting. In addition carbamates produce less pronounced CNS effects due to limited passage through the blood brain barrier (BBB).1 Another differential diagnosis is heat stroke (especially among farmers or insect fighters during the summer season); The following table 1 summarizes differences between OP/ Carbamate poisoning and heat exhaustion signs and symptoms. l Table 1.Signs and symptoms of heat exhaustion versus OP/ Carbamate Heat ExhaustionOP / Carbamate Nausea Nausea and diarrhea Dry membranes Moist membranes Dry mouth Salivation No tears Tears Fast pulse Slow pulse Dilated pupils Small or pinpoint pupils Decontamination Healthcare professionals are at risk of poisoning during initial stabilisation of patients poisoned with organophosphorus. There are some case reports for such poisoning; fortunately none have shown inhibition of acetylcholinesterase or butyrylcholinesterase in health-care workers consistent with substantial exposure to organophosphorus. Therefore, rescuers should wear chemicalprotective clothing and gloves when handling a grossly contaminated victim. If there is heavy liquid contamination with a solvent such as xylene or toluene, clothing removal and victim decontamination should be carried out outdoors or in a room with high-flow ventilation. Guidelines also recommend universal precautions, maximum ventilation, and frequent rotation of staff, so that effects of solvent and pesticide are kept to a minimum.5 Skin: remove all contaminated clothing and wash exposed areas with soap and water. Eyes: irrigate with generous amount of slightly warm water. Ingestion: activated charcoal may be given with early presentation and when the patient is cooperating. Gastric lavage should be done only after intubation. Dialysis is of no value most of the times due to large volume of distribution of OPs. s Extemporaneou Treatment6 Treatment includes emergency and supportive treatment; secure open airway, give oxygen, if intubation is required, use non- depolarizing agent such as atracurium or vecuronium. Give benzodiazepine for seizures, it also helps if patient is agitated. Specific drugs and antidotes; Muscarinic antagonist (Atropine) and AChE activator such as pralidoxime chloride. Atropine dose: If symptomatic patient, intravenous (IV) atropine should be given until atropinization is achieved. For adults a dose of 2 to 5 mg; and for children a dose of 0.05 mg/kg is recommended. If inadequate response, double the dose and repeat it every 10 to 20 minutes as needed to dry pulmonary secretions. Atropinization may be required for hours to days depending on severity. Despite of the controversy regarding the effectiveness of pralidoxime7, it is recommended by the WHO guidelines and almost in all textbooks, however there is no consensus on the optimum dose. Pralidoxime Chloride dose: WHO currently recommends an initial bolus of at least 30 milligrams/kilogram followed by an infusion of more than 8 milligrams/kilogram/hour. Alternatively adults may receive 1 to 2 g pralidoxime in 100 milliliters of 0.9% saline to be infused over 15 to 30 min. Follow by infusion of 500 mg to 1 g/hr as a 2.5% solution. Alternatively, the initial dose may be repeated 1 hr and then every 3 to 8 hr if muscle weakness or fasciculations persist (continuous infusion is preferred). Children may receive initially 20 to 50 mg/kg (max 2 g/dose) infused over 30 minutes as a 5% solution in 0.9% saline, followed by a continuous infusion of 10 to 20 mg/kg/hour. Alternatively, repeat initial bolus dose in 1 hr and then every 3 to 8 hr if muscle weakness or fasciculations persist (continuous infusion preferred). References: 1. Michael Eddleston, N.A.B., Peter Eyer, Andrew H Dawson, Management of acute organophosphorus pesticide poisoning. Lancet, 2008. 371: p. 597-607. 2. David A. Tanen, M., Poisoning & Drug Overdose, in Organophosphorus and Carbamate Insecticides, K.R. Olson, Editor. 2006, McGraw-Hill. 3. WHO | The WHO Recommended Classification of Pesticides by Hazard. 2004. 4. Toxicology Secrets. The Secret Series, ed. C. Ling, Erikson, Trestrail 2001, Philadelphia: Hanley & Belfus, INC., Medical Publisher. 5. Little, M. and L. Murray, Consensus statement: Risk of nosocomial organophosphate poisoning in emergency departments. Emergency Medicine Australasia, 2004. 16(5/6): p. 456-458. 6. ORGANOPHOSPHATES in POISINDEX® Managements, in Thomson Healthcare. 2008, Thomson Reuters. 7. Bairy KL, V.S., Sharma Alok, Sammad V, Controversies in the management of organophosphate pesticide poisoning. Indian Journal of Pharmacology, 2007. 39(2): p. 71-74. Tacrolimus in oral suspension form Tacrolimus (Prograf®) is a drug that suppresses the immune system and is used to prevent rejection of transplanted organs. It accomplishes its’ immune-suppressing effect by inhibiting an enzyme (calcineurin) crucial for the multiplication of T-cells, cells that are vital to the immune process. The use of oral tacrolimus allows transplantation specialists to reduce the dose of steroids which are also used to prevent rejection. This “steroid-sparing effect” is important because of the many side effects that can occur when larger doses of steroids are used for a long period of time. Tacrolimus was approved by the FDA in April, 1994 for liver transplantation and also has been used in patients for heart, kidney, small bowel, and bone marrow transplantation.1 Tacrolimus suspension is used in most paediatric hospitals and can be compounded at a concentration of 0.5 mg/mL. The existing 0.5mg/mL suspension has a two-month expiry date, requiring frequent manufacture.2 Because of the potential risk of toxicity, it is prudent to avoid unnecessary exposure through either skin contact or inhalation of dust. Precautionary measures such as using a fume (or vertical-laminar-airflow) hood and wearing latex gloves are recommended. If a fume hood is not available, it is advised to wear a surgical mask while at the same time minimizing the creation of dust.4 Table 1 is one method for the preparation of Tacrolimus suspension at a concentration of 0.5 mg/ml. 3,4 Table 1. Ingredients Tacrolimus (capsules) ----------------- 25 mg Ora-Plus suspending agent ----------------- 25 mL ® Simple Syrup, NF QSAD ------------- 50 mL Final concentration = 0.5 mg/mL Method 1. Empty the contents of five 5mg Prograf® capsules into a glass mortar and work to a smooth paste with a small portion of the Ora-Plus® suspending agent. 2. Add Ora-Plus® gradually and mix well until all the 25 mL of Ora-Plus® are added. 3. Add about 25 mL of Simple Syrup NF to bring the total volume of the suspension to 50 mL 4. Mix well and transfer to an amber polyethylene terephthalate glycol (PETG) bottle. 5. Label the bottle “Shake Well Before Using” and “Use Cytotoxic Drug Precautions.” 6. Label with an expiration date of 50 days. References: 1. “TACROLIMUS-ORAL Index “ through online :http://www.medicinenet.com/ tacrolimus-oral/article.htm , Accessed on 27th August ,2009 2. Australian Resource Centre for Healthcare Innovations (ARCHI) , http://www. archi.net.au/e-library/safety/tacrolimus , Accessed on 27th August ,2009 3. Tacrolimus Extemporaneously Prepared, http://www.umm.edu/altmed/drugs/ tacrolimus-121250.htm , Accessed on 27th August ,2009 4. Extemporaneous Preparations , http://the Drug Monitor - Extemporaneous Preparations - Nasr Anaizi, PhD.mht , Accessed on 27th August ,2009 Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 I 8 Is the use of Oral hypoglycemic agents contraindicated during pregnancy? I nsulin use during pregnancy is preferred over oral hypoglycemic agents (OHAs) based on the perceived idea that insulin does not cross the placenta. However OHAs cross the placenta causing stimulation of the fetus pancreas leading to hypoglycemia.1 Furthermore, early reports of increased mortality with the use of some OHAs compared with insulin discouraged the use of OHAs in the management of gestational diabetes.2 Recent studies have shown that not all OHAs cross the placenta equally. While some of them cross freely, others cross minimally. Sulfonylureas In one experimental study, four members of sulfonylureas were tested for placental transfer using the human placental cotyledon perfusion model. The investigators found that tolbutamide and chlorpropamide more readily pass the placenta (21.5% and 11% respectively) than glyburide (glibenclamide) and glipizide (3.9% and 6.6%) respectively.3 Evidence from another study showed that glibenclamide does not cross the placenta. The study in which serum cord blood in 12 pregnant women was tested for the presence of glibenclamide, the women were taking glibenclamide with average dose of 9 mg/day, although the drug was detected in the maternal serum, it was not detected in the serum cord blood. The absence of glibenclamide at the serum cord blood was explained by the author to be due to the high protein binding of glibenclamide (99.8%) and its short elimination half life 10 hours.4 Metformin has been shown to pass freely across the placenta.5 Two in vivo studies measured maternal and cord blood samples in women taking metformin throughout pregnancy (850 mg twice daily in 15 women and 2000 mg/day in 8 women). The results of these trials showed that the fetus is exposed to concentrations as high as or higher than those seen in the maternal circulation.5’6 The relatively new class, thiazolidinedione was also found to cross the placenta during the first trimester in one study. The study included 31 women who were undergoing surgical terminations at 8-12 weeks of gestation; all women were given two 4 mg doses of rosiglitazone. Analysis of fetal tissue, coelomic fluid, and amniotic fluid revealed that rosiglitazone crossed the placenta in all women, especially in those over 10 weeks’ gestation7. OHAs during first trimester The safety of glibenclamide at doses 5-20 mg daily and metformin at doses 1.5-3g daily during the first trimester was studied in several small cohort studies, only two of them showed an increased rate of congenital anomalies compared with women taking insulin. However, these two studies were small (20 and 43) respectively; 9 i Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 women did not have ideal glycemic control and a regression analysis was not performed in either study to control for glycemic control.5 Sulfonylureas were not found to be associated with congenital anomalies in another well controlled study. The study included women with type 2 diabetes mellitus who were treated with diet therapy, exogenous insulin, or sulfonylurea compounds during the first 8 weeks of gestation. Assessment of all the delivered infants (332) in this study showed no effect for the treatment modality on the rates of both major and minor anomalies. These results lead to the conclusion that the risk in individual patients appears to be related to maternal glycemic control rather than to the mode of antidiabetic therapy during early pregnancy.8 In one study, metformin (2.55 g/day) was used to facilitate conception in 72 oligoamenorrhoeic women with polycystic ovary syndrome (PCOS). In the 63 live births, there was no difference between them and the normal neonatal population at the primary outcomes. Metformin therapy was safely associated with reduction in first trimester spontaneous abortion and in gestational diabetes, was not teratogenic, and did not adversely affect birth-weight or height and motor and social development at 3 and 6 months of life.9 In another retrospective study, sixty-five women with polycystic ovary who received metformin and became pregnant during treatment were compared with 31 women who did not receive metformin (control group). The early pregnancy loss rate in the metformin group was significantly lower than the control group (P < 0.001). In the metformin group, 62 pregnancies resulted in live births. Of these, 53 were term deliveries and 8 were preterm (<37 wk). All babies were normal neonates with appropriate size for gestational age. Only one baby, delivered at term, demonstrated a fetal abnormality (achondrodysplasia). In the control group, 18 pregnancies resulted in live births. Of these, 12 were term deliveries and 6 were preterm. No fetal abnormalities occurred in the placebo group.10 The published data on the use of thiazolidindione during the first trimester is sparse, a case report described a 25-year-old woman with PCOS who took rosiglitazone 4 mg/day for the first 4 weeks of the pregnancy and delivered a healthy infant.11 All the above data about the use of OHAs during the first trimester are not from randomized controlled trials (RCT); a more robust data from properly conducted randomized controlled clinical trials are warranted, however, it appears that both sulfonylureas and metformin have low risk for teratogenecity. OHAs during second and third trimesters Glibenclamide use during the second and third trimesters was studied in a randomized controlled trial (RCT) that included 404 women with gestational diabetes that required treatment. The women were randomly assigned between 11 and 33 weeks of ges- NTH QUESTION OF THE MO tation to receive glibenclamide or insulin. The primary end-point was achievement of the desired level of glycemic control. Secondary end-points included maternal and neonatal complications. In the two groups there were significant reductions in the glucose levels from baseline in both groups, however the inter group difference between glibeclamide and insulin was not significant, (Table 1). Furthermore, there were no significant differences between the glibenclamide and insulin groups in all safety parameters for secondary end point, (Table 2). The cord-serum insulin concentrations were similar in the two groups, and glibenclamide was not detected in the cord serum of any infant in the glibenclamide group. The authors of the study concluded that in women with gestational diabetes, glibenclamide is a clinically effective alternative to insulin therapy.12 Table 1. Primary Endpoint GlibenclamideInsulin Pretreatment During treatment Pretreatment During treatment 114±19 105±16 116±22 105±18 The mean (±SD) blood glucose concentration (mg/dL) (P=0.99) Table 2. Secondary Endpoint Safety parameterGlibenclamideInsulin The percentage of infants who were large for gestational age 12% 13% Incidence of macrosomia, defined as a birth weight of 4000 g or more 7% 4% Lung complications 8% 6% Neonatal hypoglycemia 9% 6% Number admitted to neonatal intensive care unit 6% 7% Incidence of fetal anomalies 2% 2% Conclusion: Insulin therapy is effective in achieving the appropriate levels of glycemic control, but it is inconvenient and expensive. Oral hypoglycemic agents are more convenient, improve the adherence and are cheaper alternatives. Among oral hypoglycemic agents it was found that: while metformin, rosiglitazone, and most sulfonylureas cross the placenta, glibenclamide on the other hand has been shown to cross very little. Furthermore glibenclamide was found to be as clinically effective and safe as insulin in glycemic control during the second and third trimesters in a large and well designed RCT. Further studies are needed to confirm the role of metformin and thiazolidinedione in the management of gestational diabetes. References: 1. Moretti, M.E., M. Rezvani, and G. Koren, Safety of Glibenclamide for Gestational Diabetes: A Meta-Analysis of Pregnancy Outcomes. Ann Pharmacother, 2008. 42(4): p. 483-490. 2. Feig, D.S., G.G. Briggs, and G. Koren, Oral Antidiabetic Agents in Pregnancy and Lactation: A Paradigm Shift? Ann Pharmacother, 2007. 41(7): p. 1174-1180. 3. Elliott, B.D., et al., Comparative placental transport of oral hypoglycemic agents in humans: a model of human placental drug transfer. American Journal Of Obstetrics And Gynecology, 1994. 171(3): p. 653-660. 4. Kraemer, J., et al., Perfusion studies of glibenclamide transfer across the human placenta: implications for fetal safety. American Journal Of Obstetrics And Gynecology, 2006. 195(1): p. 270-274. 7. Chan, L.Y.-S., J.H.-k. Yeung, and T.K. Lau, Placental transfer of rosiglitazone in the first trimester of human pregnancy. Fertility and Sterility, 2005. 83(4): p. 955-958. 8. Towner, D., et al., Congenital malformations in pregnancies complicated by NIDDM. Diabetes Care, 1995. 18(11): p. 1446-1451. 9. Glueck, C.J., et al., Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum. Reprod., 2002. 17(11): p. 2858-2864. 10.Jakubowicz, D.J., et al., Effects of Metformin on Early Pregnancy Loss in the Polycystic Ovary Syndrome. J Clin Endocrinol Metab, 2002. 87(2): p. 524529. 5. Vanky, E., et al., Placental passage of metformin in women with polycystic ovary syndrome. Fertility and Sterility, 2005. 83(5): p. 1575-1578. 11. Nicholas, A.C., et al., Improvement in insulin sensitivity followed by ovulation and pregnancy in a woman with polycystic ovary syndrome who was treated with rosiglitazone. Fertility and Sterility, 2001. 76(5): p. 1057-1059. 6. Charles, B., et al., Population Pharmacokinetics of Metformin in Late Pregnancy. Therapeutic Drug Monitoring, 2006. 28(1): p. 67-72. 12.Langer, O., et al., A Comparison of Glibenclamide and Insulin in Women with Gestational Diabetes Mellitus. N Engl J Med, 2000. 343(16): p. 1134-1138. Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 I 10 Minimizing adverse drug events in elderly Introduction: A dverse drug events (AEs) in the elderly are of great concern in healthcare today because of a combination of ageing populations and the tendency to prescribe for every disorder experienced by patients. These AEs do not only have a direct impact on patients in the form of loss of trust, morbidity and mortality but also on healthcare professionals in the form of stress and they pose an enormous financial burden on society. This may be due in part to the fact that most common chronic diseases are prevalent in the elderly, but the wide spread use of drugs such as diuretics, CNS agents and non steroidal anti-inflammatory drugs, suggest that excess prescribing is a continuing problem.2 Drug utilization data shows that the elderly are major drug consumers, which suggests that over prescribing is common in elderly.1 One study conducted in the United Kingdom showed that elderly over 65 comprise 12%-18% of the population; receive up to 40% of all prescriptions. The study also showed that almost two-thirds of elderly may be taking either prescribed or over the counter preparations at any one time. 1. Exaggerated physiological effects of drugs due to age related pharmacokinetics changes e.g. reduced renal and hepatic clearance. 2. Co-morbidity resulting in the possibility of drug-disease interactions. 3. Poly-pharmacy drug-drug interactions. 4. Patient non-adherence possibly due to frailty, reduced dexterity and memory problems.3 The elderly (i.e. those aged 65 or older) are at increased risk of ARs due to the following reasons: Due to excessive prescribing or over-use of medicines by the elderly, they experience a disproportionate share of unwanted and unexpected adverse drug events. In a study published in the US, it was reported that about one in three older persons taking at least five medications will experience an adverse drug event each year, and about two thirds of these patients will require medical attention.4 Approximately 95 percent of these reactions are predictable, and about 28% are preventable.5 Epidemiological studies showed that there are certain drug classes most commonly associated with adverse drug events in elderly, including: diuretics, beta-blockers, warfarin, selective serotonin reuptake inhibitors, angiotensin-converting enzyme (ACE)-inhibitors and non-steroidal anti-inflammatory drugs (NSAIDs). These treatments are frequently co-prescribed in this population, thereby greatly increasing the risk.3 Remember Ask your patients to bring all their medications including prescription only, over the counter (OTC), herbal and homeopathic remedies at every visit. 11 i Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 PHARMACOVIGILANC E Table 1 Practical advice for clinicians when prescribing for elderly6 Recommendation Examples Advantages Maximize the dose of one agent for a specific disease before adding a second agent Anti-hypertensive and anti-diabetic agents Less confusion and cost, less risk of severe drug reactions Use one agent to treat two conditions: reduce poly-pharmacy Drugs used to treat hypertension and benign prostatic hyperplasia, hypertension and angina Less confusion and cost, less risk of adverse events Use an agent no more than once or twice daily Antihypertensive agents, such as ACE inhibitors, calcium channel blockers, beta blockers Better compliance Avoid misuse of drugs: potentially inappropriate drugs Amiodarone, barbiturates (except Phenobarbital), methyldopa, nifedipine Less risk of adverse events Minimize the use of anticholinergic agents and discontinue when possible Phenothiazine major tranquilizers, atypical antipsychotics, narcotics, antispasmodics, sedating cold and cough medications, OTC sleep drugs, muscle relaxants. Less risk of adverse events Ask patient to bring medications and review at every visit All medications including OTC drugs. Less confusion compliance Avoid overdosing of medications Renal functions play an important role in altering the effect of a drug in elderly. Use Cockcroft-Gault equation in the absence of the 24 hrs urine measurement to calculate age adjusted estimate of the glomerular filtration rate.6 Less risk of adverse events Avoid underuse of medications Under-prescribing: rheumatoid arthritis beginning in older adulthood, depression, diastolic heart failure and ataxia secondary to normal pressure, hydrocephalus are examples of conditions that should be diagnosed and treated in older patients but are often missed. Encourage patient adherence Consider switching to combination medications or once daily dosing to improve adherence, being careful to balance improved convenience with increased cost. Combine cognitive aids and patient education to improve adherence such as tablet dispenser boxes. Conclusions Potentially preventable medication-related adverse events have received increasing attention, in particular those, which are the result of medical errors. The elderly are at elevated risk due to physiological decline, co-morbidity and poly-pharmacy, resulting in particularly high rates of hospital admissions and death due to potentially preventable medication-related adverse events. Physicians have to find ways to streamline the medical regimen, such as periodically reviewing all medications and avoiding new prescriptions to counteract adverse drug reactions. The use of drug interaction program or computerized alerts is very beneficial to reduce medication errors and adverse drug reactions.3 better Patient compliance References: 1. Cartwright A, Smith C. Elderly people, their medicine and their doctors. London: Routledge, 1988;39-76. 2. Prescribing in the elderly. Ken Woodhouse. J Hong Kong Med Assoc Vol. 43, No. 4, Dec. 1991; 205-208 3. Kathrin M. Cresswell, Bernard Fernando, Brian McKinstry and Aziz Sheikh. Adverse drug events in the elderly. British Medical Bulletin 2007 83(1):259-274 4. Hanlon JT, Schmader KE, Koronkowski MJ, Weinberger M, Landsman PB, Samsa GP, et al. Adverse drug events in high risk older outpatients. J Am Geriatr Soc 1997;45:945-8. 5. Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003;289:1107-16. 6. Drug therapy in elderly patients: how to avoid adverse effects and interactions. http://www.consultative.com/display/article/10162/40114 Accessed on May 17 2009 7. Cockcroft-Gault equation, http://www.nephron.com/cgi-bin/CGSI.cgi. Accessed on May 17 2009. Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 I 12 ELECTRONIC PRESCRIBING: Benefits versus Risks At A Glance: 1. PLANNING PHASE: lectronic prescribing (e-Prescribing) is the utilization of electronic systems to facilitate and enhance the communication of a prescription of medicine order, aiding the choice, administration and supply of a medicine through knowledge and decision support and providing a robust audit trails for the entire medicines use process. Prior to drafting an e-Prescribing strategy, all potential benefits versus risks need to be considered. Later, the weight of each identified barrier has to be tied (practically) in to an effective solution prior to implementation. This workout can be summarized in 3 steps: E e-Prescribing has been heralded as bringing major benefits to patients by reducing the incidence of medications errors and improving communication about medicines.1 It will also support clinical activity by interacting with knowledge sources and providing decision support at the point of prescribing or administration. On the other hand, e-Prescribing is a critical component in the overall strategy to enhance health care quality. By delivering clear and accurate information throughout the prescribing process, ePrescribing can help reduce the estimated 3 million preventable adverse drug events and reduce the estimated 7,000 deaths from medication error that occur each year in the US.2 In consequence, e-Prescribing will impact on and require the support of everyone who prescribes, supplies or administers medicine. In addition to doctors and hospital pharmacists, this will include nurses, midwives, and allied health professionals such as radiographers and physiotherapists. Many other employees will also have an interest in the implementation of ePrescribing - from senior management teams within a health care facility (HCF) to the information technology teams which will provide local support to the systems. PHASES OF E-PRESCRIBING IMPLEMENTATION: The implementation of e-prescribing will require new roles in health/pharmacy informatics to support the continued development of systems based on feedback. There will be changes to practice and evidence generated that will enhance medicines management, risk reduction, patient safety and quality improvement in a variety of ways. The systems will facilitate the supply of medicines through links to stock control and automation. Time released from “traditional” activities will need to be redirected to clinical activities and used to promote optimized treatment for individual patients. Illustrated below, are the different phases of implementing e-Prescribing. STEP 1: Identifying Benefits of Implementing e-Prescribing: Literature revealed 3 categories of possible benefits: 1.1. Operational Benefits l Prescription always available at point of need and at multiple sites, saving staff time.2 2 l Facilitate compliance with policies and formulary. l Accurate record of all drugs administered l Ability to target clinical pharmacist activity to patients with greatest need. l Ability to quickly identify high risk patients l Reduce transcription errors prescriptions l Allergy warnings always available and linked to drug selection l Reduce adverse drug events l Identifies drug-drug interactions at the point of prescribing 1.2. Managerial Benefits 2 l Promote rational use of medicines. l No legibility issues 2 l Information on drug availability at the point of prescribing. l Notes can be attached clarifying decisions l Reduce drug selection errors and ability to retrieve previous medication history for re-admissions.3 3 l Reduce dose, frequency and duration errors. l Reduce risk of administration errors through bar code recognition at point of administration.3 l Reduced delays in treatment by reducing delays from prescribing to supply l Reduce missed doses as prescription available at all times l Ability to track and audit changes in drug treatment during admission l Potential to link with other decision support systems e.g. pathology that influence choice of drug, dose etc at the point of prescribing.4 l Ability to restrict the prescribing of high risk drugs to specific clinicians.4 l Accurate medication histories able to be transmitted to GPs including changes to therapy.4 1.3. Financial Benefits l Ability to accurately cost drug treatment of patients down to what has been administered rather than what has been supplied.4 l Ability to accurately track drugs excluded from insurance plan- for recharge purposes. l Reduced cost of dealing with adverse drug events. 13 i Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 REVIEW Save staff time looking for and accessing drug charts by medical, nursing and pharmacy staff. l Save staff time transporting drug charts around the site e.g. ward to pharmacy. l Potentially some drug expenditure savings through tighter control of prescribing.2 l STEP 2: Identifying risks of implementing e-Prescribing 2.1.Operational Risks l Service delivery if system fails l Does not cover all prescribing e.g. chemotherapy l Incomplete use of system and therefore records if access is not optimized, requires access to appropriate IT hardware e.g. laptops, tablets, mobile PCs, etc.5 No. Barrier 2.2. Managerial Risks l Need for manual backup system and procedures l Staff deskilled in the use of hard copy 2.3.Financial Risks 5 l Initial capital outlay including the provision of portable units. l Revenue costs for license l Staff to support the system STEP 3: Adjusting e-claim strategy to accommodate identified barriers and risks of implementation: Prior to implementation, we need to customize e-Prescribing strategy to surmount the identified barriers practically. The table below summarizes possible strategy remodeling to accommodate latent barriers. Strategy 1 Unclear goals for e-Prescribing conversion Identify clear measurable goals to be achieved through e-Prescribing 2 Magnitude of change overwhelms the targeted HCPs/HCFs* Consider phased implementation (e.g.: implement e-Prescribing for refills first, follow with addition of e-Prescribing for new prescriptions , or implement e-prescribing to inpatient then to outpatient , tertiary then to PHC..etc) 3 HCPs/HCFs may lack technical skills to implement e-Prescribing Survey HCPs/HCFs to confirm mastery of basic computer/typing/change management skills 4 Confusion as to how to integrate e-Prescribing into current work flow Conduct workflow analysis of current prescribing workflow including time study 5 HCPs/HCFs anxiety regarding change process or even job security Appoint an in-house project manager. Engage staffs who perform prescription management activities to help document workflows. Clearly communicate benefits of e-Prescribing to the practice. 6 Lack of XYZ contribution Appoint representatives from XYZ to be involved in choosing, implementing and evaluating e-Prescribing system 7 E-Prescribing system does not support critical percentage of patient population Make sure the e-Prescribing system chosen is compatible with majority of participating payers 8 Learning curve for new system underestimated Maintain flexible implementation timeline. Use vendor’s experience for training/implementation learning curve estimate 9 New system does not perform on “Day One” Test software including pharmacy interface prior to go-live. Take typical day prescription volume and do a dry run in a test database. Ask vendor if any bugs exist in current release – if so ask about dates for patches or upgrades 10 Hardware does not operate correctly Test all hardware in advance of go-live. Make sure staff/physicians are familiar with operation of any new devices 11 Inadequate time and resources for training. Allow adequate time for training outside of clinical work sessions. Appoint “super users” to assist staff and physicians. Assess team readiness prior to committing to go-live date 12 HCPs/HCFs unprepared to fully utilize new system Schedule go-live soon after training. Consider short-term reduction of patient volume. Make sure “super users” are available. Confirm availability of vendor support services 13 New process is not fully implemented Monitor outcome measures and share with HCPs/HCFs. Ask for feedback and quickly address problems as they are identified. Offer follow-up training sessions. Confirm vendor support of training/ upgrades/ service support * Note: HCPs/HCFs refer to that the strategy aims to cover References: 1. The Institute of Medicine(US). To Err is human: building a safer health system. National Academy Press 1999 2. Hillestad R et al. Can Electronic Medical Record Systems Transform Health Care? Potential Health Benefits, Savings, and Costs, Health Affairs, 2005; 24(5):11031117. 3. Institute of Medicine, Preventing Medication Errors, National Academies Press, Washington, DC, 2006. 4. Fischer, et. al., “Effect of Electronic Prescribing With Formulary Decision Support on Medication Use and Cost,”Archives of Internal Medicine, 168 (22), 2008, pages 2433-2439. 5. E-Prescribing’s Impact on Cost and Quality: Implications for Pay-for-Performance Initiatives. Presentation by Leo Barbaro, Vice President, Anthem BCBS Connecticut, Health Information Technology Summit, March 2005. McMullin ST, Lonergan TP, Rynearson CS, et al. Impact of an evidenced-based computerized decision Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 I 14 Insulin Glargine (LANTUS®) & Cancer - A Closer Look Background O n Friday, June 26, 2009, the EASD (European Association for the Study of Diabetes) released on its website and in its journal ‘Diabetologia’ a series of papers, an editorial, a press release, a video statement, and patient information, all concerning a “possible link between insulin glargine and cancer” based on four observational studies and a randomized controlled trial.1 Other international organizations quickly followed by releasing their own position statements. The American Diabetes Association advised patients using insulin not to stop taking it based on the findings of the studies.2 The American Association of Clinical Endocrinologists (AACE) did not recommend that the use of any insulin be changed.3 The Endocrine Society and The Hormone Foundation, the public education affiliate of The Endocrine Society, recommended that patients continue with their current insulin therapy until they have discussed with their physicians the reasons why a particular insulin treatment was prescribed.4 Regulatory agencies also released position statements. The FDA and Health Canada recommended that patients should not stop taking their insulin therapy without consulting a physician, since uncontrolled blood sugar levels can have both immediate and long-term serious adverse effects.5,6 EMEA, on the other hand, stated that the available data did not provide a cause for concern and that changes to the prescribing advice were therefore not necessary. 7 The World Health Organization (WHO) estimates that more than 180 million people worldwide have diabetes.8 They also state that the number is likely to more than double by the year 2030.8 In light of these statistics, it was no surprise that the recent warnings about insulin glargine (LANTUS®) raised such a global uproar. Literature Review 1. According to the authors of the editorial, Insulin Glargine and Cancer – An Unsubstantiated Allegation, none of the studies showed a relationship between insulin glargine and cancer. 1 2. The German study found that there is a decrease in both cancer risk and all-cause mortality when insulin glargine is compared to human insulin; 1,9 3. The Swedish study found no increase in overall cancer risk with insulin glargine, but for breast cancer there was an increased risk with use of insulin glargine alone, but not in combination with other insulins, and with a decrease in allcause mortality; 1,10 4. The Scottish study found no increase in overall cancer risk with insulin glargine and uninterpretable data with regard to breast cancer; 1,11 15 i Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 5. The UK THIN study found no increase in overall cancer risk and no increase in breast cancer risk with insulin glargine but did find an increase in risk with insulin and insulin secretagogues versus metformin; 1,12 6. A small randomized study found no increase in cancer risk.1 A strength of this trial was that it was randomized (thereby eliminating distribution bias); however, its shortfall was that it was small.1 Discussion Since none of the studies showed a relationship between insulin glargine and cancer, why were some patients advised to see their healthcare providers anyway? The answer to this question may not be as simple. It may be because individual study data are not fully understood or expert agencies are in the process of reviewing the data or, perhaps, the data collectively has simply shaken confidence in a product once considered to be very safe and effective – leaving experts confused. The most likely answer may be a combination of reasons. This is why a call for trial data to examine if a risk actually exists has been resonating from every corner. A randomized trial may be the best way to establish or refute whether a relationship between insulin glargine and cancer exists.1 Currently, discussions are ongoing between FDA and the manufacturer of insulin glargine as to whether any additional studies evaluating the safety and efficacy of this drug will need to be performed. A large randomized trial has been underway since 2003, the ORIGIN (Outcome Reduction with an Initial Glargine Intervention) trial.1 Examination of the frequency of cancer in ORIGIN may help resolve the question; does insulin glargine have a similar (or decreased) rate of cancer in comparison to the control group.1 However, if there is an increased rate of cancer in the insulin glargine group, it will be impossible to distinguish whether that increase is due to insulin use or insulin glargine use.1 Moreover, given the observation that metformin use may result in decreased cancer risk, the use of metformin may complicate analysis and make interpretation difficult.1 In other words, if metformin was used only in the standard therapy group, then a reduction in cancer risk in that group when compared to the control therapy group might have shown a false statistical increase in cancer risk in the control therapy group. If both control and standard therapy groups received metformin, then even if a cancer risk existed, sensitivity analysis might not detect a difference. Scenario in UAE In UAE, approximately 19.5% of the population has either type I or type II diabetes,8 a higher incidence rate when compared to the rest of the developed countries. In addition, according to Medication Safety the WHO, incidence of diabetes and obesity are on the increase in the UAE.8 Obesity and type II diabetes by themselves are associated with an increased risk of cancer.1 2. The American Diabetes Association Website. http://www.diabetes.org/for-media/ pr-glargine-0602609.jsp. Accessed date: October 1, 2009. Differences between the UAE population and individuals in the studies published in Diabetologia and in the small randomized trial do exist. Other differences such as sedentary lifestyle, which is a major risk factor for diabetes, is a related factor that may be worth considering when trying to extrapolate the data to the UAE population. 4. The Endocrine Society Website. http://www.endo-society.org/advocacy/policy/ upload/Statement-for-Patients-on-Insulin-Glargine.pdf. Accessed date: October 1, 2009. Would statistical power, clinical significance, number needed to harm be any different if the study subjects were taken from the UAE population? A weakness of the Scottish study (which is acknowledged by the authors) was allocation bias; thereby, those patients who were less healthy were treated with insulin glargine.1,11 The average age of the patients in the German study was 69.5 years.1 Would allocation bias be eliminated in a population that was prescribed insulin glargine for a wider age group and during all stages of the disease? In other words, would replacing the subjects in the observational studies and randomized trial with different subjects (without any other changes to the designs) simply correct several of the limitations of the studies? For the time being, we simply do not have the answer to this and others questions. 3. The American Association of Clinical Endocrinologists (AACE) Website. http:// www.aace.com/. Accessed date: October 1, 2009. 5. Food and Drug Administration. www.fda.gov. Accessed date: September 15, 2009. 6. Health Canada. www.healthcanada.com. Accessed date: September 15, 2009. 7. European Medicines Agency. http://www.emea.europa.eu/. Accessed date: September 15, 2009. 8. World Health Organization. http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed date: August 20, 2009. 9. Hemkens LG, Grouven U, Bender R, Gu¨ nster C, Gutschmidt S, Selke GW, Sawicki PT: Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009; doi 10.1007= s00125-009-1418-4. 10. Jonasson JM, Ljung R, Talba¨ck M, Haglund B, Gudbjo¨ rnsdo` ttir S, Steineck G: Insulin glargine use and short-term incidence of malignancies—a population-based follow-up study in Sweden. Diabetologia 2009; doi 10.1007=s00125- 009-1444-2. 11. SDRN Epidemiology Group: Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009; doi 10.1007=s00125-009-1447-z. 12. Currie CJ, Poole CD, Gale EAM: The influence of glucose lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; doi 10.1007=s00125-009-1440–6. 13. Health Authority Abu Dhabi (HAAD). Circular No. PHM/29/09. July 22, 2009. Conclusion Toxicology Quiz The data implicating insulin glargine as a cause of cancer was inconclusive in the studies mentioned. However, this does not mean that an increased risk does not exist - particularly in the UAE population. Therefore, HAAD will continue to monitor the situation closely for any new developments and alert the public and healthcare professionals as necessary. 1.Of the 3,000 known species of snakes in the world, approximately what percent are venomous? HAAD Position Statement 13 The HAAD position is that treating physicians should carefully investigate their patients before prescribing LANTUS® (insulin glargine) and to report any adverse events to the HAAD Pharmacovigilance Center. References: 1. Satish K. Garg, M.D.,1 Irl B. Hirsch, M.D.,2 and Jay S. Skyler, M.D., M.A.C.P. Insulin Glargine and Cancer—An Unsubstantiated Allegation. DIABETES TECHNOLOGY & THERAPEUTICS Volume 11, Number 8, 2009. l 33 % 2.Intoxication by what element has been implicated in the pathogenesis of “Alzheimer’a disease”? l Aluminum l Lead l Mercury 3.In treating an overdose of paracetamol, an antidote called N- ACETYLCYSTEINE (NAC) is commonly used. In order to be most effective, this antidote should be given before how many hours post exposure have passed? l 4 hours l 8 hours l 12 hours 3. 12 hours European Medicines Agency’s Committee for Medicinal Products for Human Use (EMEA - CHMP) state that the available data does not provide a cause for concern and that changes to the prescribing advice are therefore not necessary. 25 % 2. Aluminum EMEA Position Statement 7 l Answers : 1. 10 % The FDA and Health Canada recommend that patients should not stop taking their insulin therapy without consulting a physician, since uncontrolled blood sugar levels can have both immediate and long-term serious adverse effects. 10 % Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 I FDA and Health Canada Position Statement 5,6 l 16 Rumors Versus Truth I’ve heard .... Sometimes facts get mis-stated ... statistics misquoted ... or efficacy exaggerated. If you’re hearing or seeing something .... that leaves you wondering if it’s really true, tell us about it. We’ll check it out for you and put the “best” Rumors here ... along with the truth. RUMOR Combining Ibuprofen and Guaifenesin can cause heart attacks in kids. TRUTH: Websites and email inboxes are flooded with claims of an 8 year-old girl dying after taking this combination. Reassure patients that this is just an Internet rumor. There is NO evidence it ever occurred...no confirming news reports, additional details, or ANY verifiable stories of the event and no serious interactions are expected between any drugs in this combination …ibuprofen, guaifenesin, dextromethorphan, etc. Tell people that problems due to OTC cough and cold products in children are usually due to misuse or accidental ingestion. If parents ask, tell them it’s safe to use Ibuprofen and guaifenesin together and there is no evidence of interaction. Reference: 1. Rumor vs Truth. Pharmacists Letter. http://www.pharmacistsletter.com- password web access. Accessed on August 25,2009 RUMOR Vitamin E interacts with statins TRUTH: Lots of patients take vitamin E and other antioxidants with the hope of preventing heart disease. There is some evidence that a high DIETARY intake of vitamin E might reduce the risk of heart disease. But the most recent evidence shows that vitamin E SUPPLEMENTS do not help prevent primary or secondary heart disease or cardiovascular events such as heart attack or stroke.2 Recent evidence suggests that taking a combination of antioxidants including vitamin E might actually be harmful. Many patients with heart disease get a statin such as simvastatin, lovastatin, atorvastatin, etc. In addition to lowering the bad LDL cholesterol, statins also increase the good HDL cholesterol. Patients who take simvastatin and niacin in combination with an antioxidant including vitamin E, vitamin C, selenium, and beta - carotene seem to have LESS of the good HDL cholesterol.3 The antioxidant combination seems to decrease HDL by about 15%. Even though vitamin E plus other antioxidants might interact with statins and decrease HDL levels, it’s too soon to say how clinically significant this might be. And there’s evidence that taking just vitamin E with a pravastatin has no adverse effect on HDL.4 17 i Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 US TR S R E V RUMORS UTH For now, think of this potential interaction as another reason to think twice about recommending vitamin E for heart disease. References: 1. Rumor vs Truth. Pharmacists Letter. http://www.pharmacistsletter.com- password web access. Accessed on August 25,2009 2. Lonn E, Yusuf S, Dzavik V, et al. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation 2001;103:919-25. 3. Brown BG, Zhao XQ, Chait A. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001;345:1583-93. 4. Carlsson CM, Papcke-Benson K, Carnes M, et al. Health-related quality of life and long-term therapy with pravastatin and tocopherol (vitamin E) in older adults. Drugs Aging 2002;19:793-805. RUMOR Artificial food additives make kids hyperactive. TRUTH: The belief that artificial food additives might be related to hyperactive behavior in children stems from over 30 years ago. A pediatric allergist by the name of Benjamin Feingold proposed that synthetic flavorings and colors in the diet might be a cause of hyperactivity, and proposed an elimination diet. Many parents who have followed the “Feingold Diet” have reported improvement in their children’s behavior.2 But evidence for a link is conflicting. Until now, most research showed no or little effect of additives on behavior.3 Now a well-designed study suggests that artificial food colorings and benzoate preservative commonly found in sweets and beverages can increase hyperactivity in kids with and without attention deficit hyperactivity disorder (ADHD).4 But experts disagree on whether the increase in hyperactive behavior is significant for most children. There are still questions on who is most susceptible and whether genetics might contribute. Plus it’s not clear whether the preservative or a particular food coloring might be the cause.5 Tell parents with “hyperactive” children that limiting additives in their child’s diet MIGHT help. But advise them that for most children, the gains are likely to be small. If they really suspect their child’s hyperactive behavior is out of the normal, refer them to their pediatrician for evaluation. References: 1. Rumor vs Truth. Pharmacists Letter. http://www.pharmacistsletter.com- password web access. Accessed on August 25,2009 2. Wolraich ML and others. Effects of diets high in sucrose or aspartame on the behavior and cognitive performance of children. N Engl J Med 1994;330:301. 3. McCann D, Barrett A, Cooper A, et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, doubleblinded, placebo-controlled trial. Lancet 2007;370:1560. 4. Bateman B, Warner JO, Hutchinson E, et al. The effects of a double blind, placebo controlled, artificial food colourings and benzoate preservative challenge on hyperactivity in a general population sample of preschool children. Arch Dis Child 2004;89:506. 5. Schab DW, Trinh NT. Do artificial food colours promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. J Dev Behav Pediatr 2004;25:423. FORMULARY UPDATE Formulary Additions - New Medications Drug Name Dosage Approved HAAD Indications Comments Pegaspargase 750 units/mL, 5 mL vial • Acute lymphoid leukemia, First-line, in combination with other agents • Acute lymphoid leukemia, In combination with other agents in patients with hypersensitivity to L-asparaginase Insulin Glulisine 100 units / ml prefilled pens & vials • Diabetes mellitus type 1 • Diabetes mellitus type 2 Telmisartan 40 & 80 mg tablets • Hypertension Oseltamivir 75 mg capsule • Influenza, Viruses Types A and B; Treatment • Influenza, Viruses Types A and B; Prophylaxis Zanamivir 5 mg diskhaler • Influenza, Viruses Types A and B; Treatment • Influenza, Viruses Types A and B; Prophylaxis Restricted to FDA approved indication in Acute lymphoid leukemia New dose Amlodipine 10 mg tablet New Dosage form Insulin Biphasic Aspart 30/70 Insulin Glarigine 100 units/ml, 3 ml prefilled pen 100 units/ml, 3 ml cartridge Formulary Deletions Drug Name Dosage AQUA 40% /PARAFFINUM Cream AQUA 50% /PETROLATUM Cream AQUA 55%/CERAMIDE Cream AQUA 60% /GLYCERIN Cream AQUA 60% /PETROLATUM Cream AQUA 80% /BUTYLENE GLYCOL Cream TACHYDRITE & CARNALITE MINERALS Maintainer Cream TACHYDRITE & CARNALITE MINERALS Body Lotion TACHYDRITE & CARNALITE MINERALS Initiator Lotion TACHYDRITE & CARNALITE MINERALSs Scalp Gel Satisfaction Survey In an effort to improve our newsletter we appreciate your time in filling out this survey: 1.How important this newsletter to you? l Very important l Satisfactory l Not Important 2.Does the newsletter provide relevant information to your practice? l Excellent l Satisfactory l Needs Improvement 3. How satisfied are you with the overall content? l Excellent l Satisfactory l Needs Improvement 4. How satisfied are you with the quality of writing in this newsletter? l Excellent l Satisfactory l Needs Improvement Kindly fax us the completed survey to fax number: 02-444 31 25 or call us on the Toll Free Number 800424 Toxi-Drug Info - Sept. 2009 - Volume 3 - Issue 3 I 18