Functional group: group of atoms with characteristic chemical
advertisement
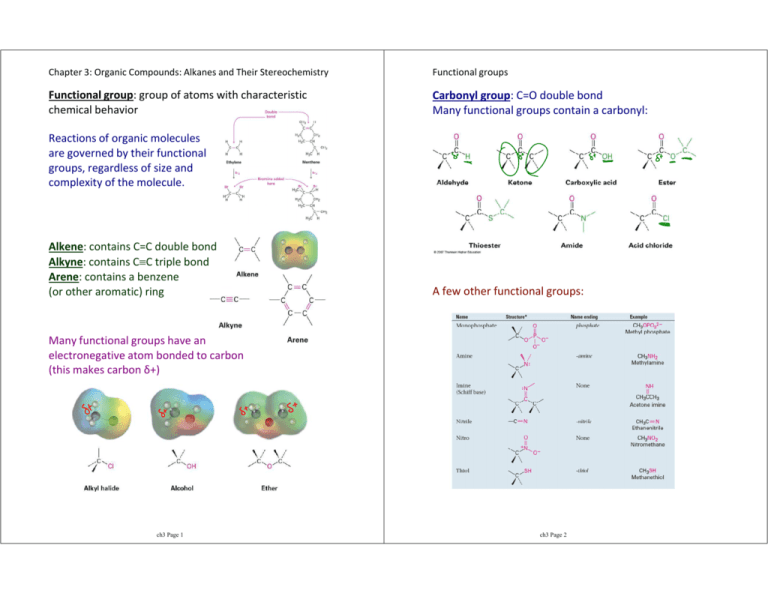
Chapter 3: Organic Compounds: Alkanes and Their Stereochemistry Functional groups Functional group: group of atoms with characteristic chemical behavior Carbonyl group: C=O double bond Many functional groups contain a carbonyl: Reactions of organic molecules are governed by their functional groups, regardless of size and complexity of the molecule. Alkene: contains C=C double bond Alkyne: contains C≡C triple bond Arene: contains a benzene (or other aromatic) ring A few other functional groups: Many functional groups have an electronegative atom bonded to carbon (this makes carbon δ+) ch3 Page 1 ch3 Page 2 3.2 Alkanes and alkane isomers Constitutional isomers Alkanes contain no functional groups - only C-H and C-C single bonds (all ____ hybridized carbons) Constitutional isomers: molecules that differ in how their atoms are connected Must have same molecular formula Methane: CH4 Ethane: C2H6 Propane: C3H8 Butane: C4H10 If an alkane has n carbons, how many hydrogens does it have? Alkanes are saturated with hydrogen (no more H's can be added) - a.k.a. aliphatic compounds. The alkanes shown above are straight-chain or normal alkanes (C's connected to no more than 2 other C's) Starting with butane, alkanes can be branched - one or more C's connect to 3 or 4 other C's) - pencil test ch3 Page 3 ch3 Page 4 Drawing alkanes Names of straight-chain (n-) alkanes The molecular formula for butane contains no structural information (C4H10) 1-4 are historical names. 5 and up are named based on the number of carbons. Memorize 1-10. Structures can be drawn out showing all bonds, but this is tedious and only used when absolutely necessary... Condensed structures can efficiently show how the atoms are connected (although no bonds are drawn)... Line structures are handy too... Common isomer names: Isobutane: Isopentane: Neopentane: ch3 Page 5 ch3 Page 6 3.3 Alkyl groups Types of alkyl groups Alkyl group: partial structure that remains after removing one hydrogen from an alkane molecule Wildcard R for any alkyl group Replace -ane with -yl in name Alkyl groups are classified by their connection site (where the hydrogen was removed from) Primary: carbon at end of chain Methane (CH4) → Ethane (CH3CH3) → Propane (CH3CH2CH3) → Secondary: carbon in the middle of a chain Tertiary: carbon attached to 3 other C's Butane → Isobutane → Primary, secondary, tertiary, and quaternary can be used to describe the location of functional groups: Pentane → Isopentane → Identify the primary, secondary, and tertiary carbons and hydrogens in the compounds below: Neopentane → ch3 Page 7 ch3 Page 8 3.4 Naming alkanes Complex structures Even complex branched organic molecules can be named using the systematic IUPAC naming process. Branching off sub-structures is handled by naming the sub-structure as if it were a compound itself: 1. Find the parent hydrocarbon chain (longest continuous chain of carbons - may turn corners!) If tied, use the chain with the most branches 2. Number atoms on the main chain (start with the end nearest to a branch point) If tied, look for the nearest 2nd branch 3. Identify and number the substituents 4. Molecule is named as a single word Combine multiple identical substituents with multiplier prefixes like di-, tri-, tetra-, etc. Alphabetize with substituent names but not multiplier prefixes Use hyphens to separate a number and a letter Use commas to separate numbers ch3 Page 9 ch3 Page 10 3.5 Properties of alkanes 3.6 Conformations of ethane Alkanes are sometimes called paraffins because they have little affinity for reactions with other substances. Stereochemistry: concerned with the 3-dimensional aspects of molecules There are 2 reactions that alkanes will readily undergo: 1. Combustion: alkanes react with oxygen to produce carbon dioxide and water, and release a large amount of heat Because σ bonds exist directly between two atoms, rotation is possible around C-C bonds in open-chain molecules. 2. Radical halogenation (which will be detailed in a later chapter): H's replaced with Cl's in the presence of strong ultraviolet light (hν) Conformations: different arrangement of molecules as a result of bond rotations Alkanes have a predictable trend in boiling and melting points due to the increase in dispersion forces: ch3 Page 11 Conformers: molecules with different conformations (conformational isomers) ch3 Page 12 3.7 Conformations of other alkanes Torsional strain The rotation about the C-C bond in ethane is not completely free rotation. Torsional strain is the barrier to rotation that causes some conformers to be more stable than others. Staggered conformation: the hydrogens on carbons 1 and 2 are as far away from each other as possible each H on C1 is 180o across from an H on C2 this minimizes torsional strain (most stable) Eclipsed conformation: the hydrogens on C1 are as close as possible to the hydrogens on C2 each H is directly in front of another this maximizes torsional strain (least stable) ch3 Page 13 Propane has similar conformations to ethane, except the torsional strain is slightly larger because one of the H-H interactions is replaced by an H-CH3 interaction. Butane is more complicated because not all staggered and eclipsed conformations have the same energy. Anti conformation: CH3 groups are 180o apart Gauche conformation: CH3 groups are 60o apart ch3 Page 14 Steric strain The additional interaction in the butane conformations is steric strain, the repulsive interaction between atoms trying to occupy the same space. Butane totally eclipsed conformation (0o): ch3 Page 15











