10.1HomologousSeriesandFunctionalGroups
advertisement

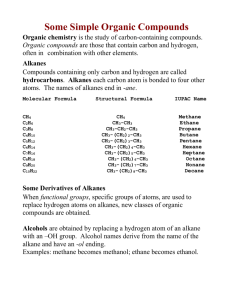
1 10.1 Homologous Series and Functional Groups Date _____________________________ 10.1.1 Describe the features of a homologous series Members of a homologous series have the same functional group and can be represented by the same general formula. Successive members of the series have an additional CH2 group The members of a series show a gradual change in their physical properties, as the chain length increases The members of a series have similar chemical properties due the presence of the same functional group within a series Example: The alkanes form a series of compounds all with the general formula CnH2n+2 Straight Chain Alkanes – An Example of a Homologous Series # Carbon Name Molecular Formula Structural Formula 1 Methane CH4 CH4 2 Ethane C2H6 CH3CH3 3 Propane C3H8 CH3CH2CH3 4 Butane C4H10 CH3CH2CH2CH3 5 Pentane C5H12 CH3CH2CH2CH2CH3 6 Hexane C6H14 CH3(CH2)4CH3 7 Heptane C7H16 CH3(CH2)5CH3 8 Octane C8H18 CH3(CH2)6CH3 9 Nonane C9H20 CH3(CH2)7CH3 10 Decane C10H22 CH3(CH2)8CH3 10.1.2 Predict and explain the trends in the boiling points of members of a homologous series. As we have already discussed, the boiling points of the alkanes increase gradually as the carbon chain length increases. [You should be able to explain this in terms of increasing vanderWaal’s] http://jchemed.chem.wisc.edu/ 2 Other homologous series are all characterized by the presence of a particular FUNCTIONAL GROUP. Use your text to identify the general formula, functional group and an example of the following homologous series. NOTE: “-R” is sometimes used to denote a hydrocarbon chain in a general formula Name of Functional General Formula or Example (Name and Structural Formula) Group Alkenes Alcohols Halogenoalkanes Aldehydes Ketones Carboxylic Acids Esters READ Brown-Ford pages 185 to 188 READ Brown-ford pages 197 and 198 and SUMMARIZE these IDEAS FOR YOUR NOTES. Try the animated tutorial mentioned on p. 197. Complete your practice nomenclature sheets for alkanes, alkenes and alkynes