Nomenclature of Alkanes
advertisement
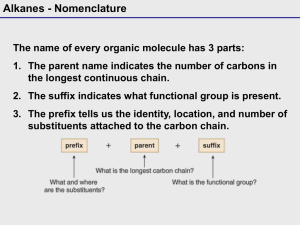
Unit 2 Alkanes and Chemical Reactions Alkanes Nomenclature Physical Properties Reactions Structure and Conformations Cycloalkanes cis-trans isomerism ring strain conformations Alkanes All C atoms are sp3. Since there are no multiple bonds, alkanes are saturated hydrocarbons. General formula for noncyclic alkanes is CnH2n+2. The noncyclic alkanes are a homologous series, differing only by the number of methylene -CH2- groups. Nomenclature We will learn the IUPAC (systematic) names of the alkanes. Below is the template you will use to build the name of ANY organic compound. stereomain functional substituents unsaturation isomerism chain group Nomenclature of Alkanes Rule #1: Name the longest continuous chain (main chain) of C atoms. When there are two chains of equal length, use the chain with the greatest number of substituents. methane CH4 hexane CH3(CH2)4CH3 ethane CH3CH3 heptane CH3(CH2)5CH3 propane CH3CH2CH3 octane CH3(CH2)6CH3 butane CH3(CH2)2CH3 nonane CH3(CH2)7CH3 pentane CH3(CH2)3CH3 decane CH3(CH2)8CH3 Nomenclature of Alkanes Rule #2: Number the main chain from the end nearest a substituent. Nomenclature of Alkanes Using the line-angle structure can be helpful in naming a compound. Nomenclature of Alkanes Rule #3: Name the substituent groups as alkyl groups. Give the location as the number of the main chain C atom to which the substituent is attached. alkyl substituents you should know methyl -CH3 t-butyl -C(CH3)3 ethyl isopropyl -CH(CH3)2 propyl -CH2CH2CH3 sec-butyl -CH(CH3)CH2CH3 butyl isobutyl -CH2CH(CH3)2 neopentyl -CH2C(CH3)3 -CH2CH3 -CH2(CH2)2CH3 Alkyl Substituents isopropyl isobutyl t-butyl sec-butyl neopentyl Nomenclature of Alkanes Rule #4: If two or more substituents are present, list them in alphabetical order. If more than one of the SAME substituent is present, use di-, tri-, tetra-, etc. Do not alphabetize a hyphenated beginning. E.g., t-butyl is alphabetized as “b”, but isobutyl is “i”. Do not alphabetize a numerical prefix. E.g., dimethyl is alphabetized as “m”. Nomenclature of Alkanes 5-ethyl-2-methyl-4-propylheptane Nomenclature of Alkanes Additional rule: If each end of the longest chain has a substituent the same distance from the end, start with the end nearer to the second branch point. Cl CH3 C H 3 C H C H C H 2C H C H 3 CH3 3-chloro-2,5-dimethylhexane Nomenclature of Alkanes Complex substituents Find the longest chain from the point of attachment. The C at the point of attachment is C #1. Name as you would an alkane, but the ending is -yl. Put the entire name in parentheses. 1-ethyl-2-methylpropyl (1-ethyl-2-methylpropyl)cyclopentane Nomenclature of Alkanes Draw the line-angle structure for the following compounds: 3, 3-dimethylpentane 4-sec-butyl-2-methyloctane 1, 2-dichloro-3-methylheptane Isomers Two or more compounds that have the same molecular formula but different arrangements of atoms. Two classes of isomers Structural isomers Molecules with different bonding patterns. Stereoisomers (Unit 3) Structural Isomers CH3CH2CH2CH3 C4H10 butane CH3 CH3 CH CH3 C4H10 isobutane IUPAC name is methylpropane Structural Isomers Draw all of the structural isomers of C6H14. Physical Properties of Alkanes Used as fuels, solvents, lubricants Nonpolar hydrophobic (water-hating) Densities of n-alkanes ≈ 0.7 g/mL Intermolecular force is dispersion (van der Waal’s), so bp’s increase with increasing surface area, as do mp’s of n-alkanes. Physical Properties of Alkanes Boiling points Straight-chain compounds will have the highest bp’s. The more branched the chain, the more compact the molecule, and the lower the bp (due to smaller dispersion attractions). Physical Properties of Alkanes Melting points of branched alkanes The effect of branching on melting point is more difficult to predict. For a given number of carbons, the more compact molecule will have the higher melting point. (Because it packs better.) Sources of Alkanes Refining of crude oil distillation followed by catalytic cracking (hydrocracking) Natural gas 70% methane, 10% ethane, 15% propane (plus small amounts of other compounds) Reactions of Alkanes The most significant reaction of alkanes is combustion. CH3(CH2)8CH3 + 15.5 O2(g) 10CO2(g) + 11H2O(l) Catalytic cracking different from hydrocracking Halogenation Structure and Conformations of Alkanes C atoms are sp3 hybridized and bond angles are 109.5°. Sigma bonds (σ bonds) end-to-end overlap rotation possible conformations differ only in dihedral angle conformations shown by Newman projections Newman Projections Pick a C-C bond and look along that axis.* * Here’s where those modeling kits come in handy. Newman Projections of Ethane Conformations Torsional Strain The eclipsed conformation has a higher energy than the other conformations, so there is some resistance to rotation. This resistance is called torsional strain (or steric strain). Ethane at room temperature has more than enough energy to overcome this resistance. Newman Projections of Butane Conformations anti is the lowest energy conformation. Conformations of Butane More distinct conformations are possible. The anti conformation has the lowest energy. This is why we draw C skeletons as zigzag lines. Gauche conformations are responsible for “kinks” in the C chain. Steric Hindrance The totally eclipsed conformation of butane has a higher energy than its other conformations due to torsional strain, aka steric strain, and sometimes called steric hindrance. The H atoms of the methyl groups are actually in each other’s way in the totally eclipsed conformation. However, butane at room temperature has enough energy to rotate through the totally eclipsed conformation.