Ionic compounds
advertisement
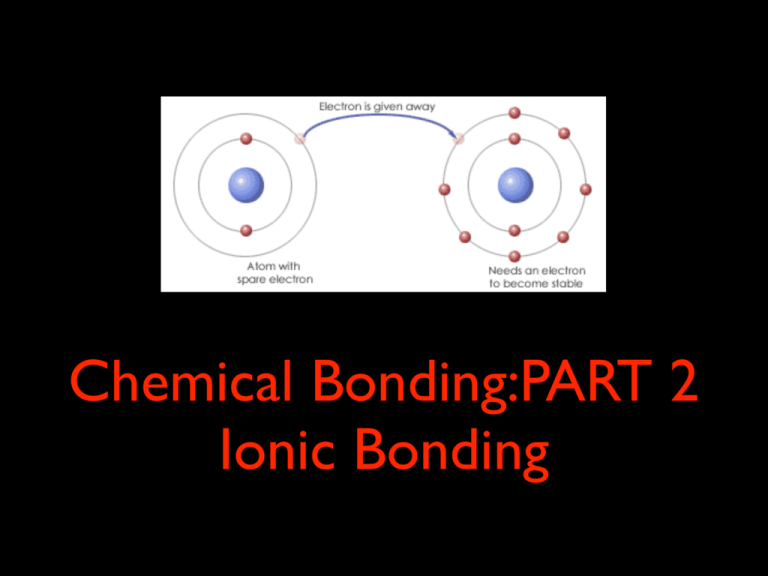
Chemical Bonding:PART 2 Ionic Bonding As you work through the following keynote, make sure you take notes in your workbook. ! By the end of the keynote, you should be able to define ionic bonding, and identify some ionic compounds. ! The tasks you will need to complete are highlighted in green text. All answers must be completed in your exercise book. ! Ionic Bonding The properties and structure of different substances depends on the way their atoms are joined together, or bonded. The way a substance reacts with other substances also depends on these chemical bonds. ! ! ! Remember that all atoms aim to achieve a stable state. An atom is stable if it has a full outer shell of electrons. ! In covalent bonding, atoms achieve full outer shells by bonding together to share electrons. ! In ionic bonding, atoms give up or take electrons from one another to achieve a full outer shell. An atom that has gained or lost electrons becomes an ion. Ions have electrical charges as they do not have an equal number of positively charged protons and negatively charged electrons. ! An atom that has lost electrons becomes a cation. It has a positive charge. ! An atom that has gained electrons becomes an anion. An anion has a negative charge because it has more electrons than protons. ! Ionic bonds are created between cations and anions. By losing and gaining electrons, these atoms become stable. TASK: Answer the following questions in your workbook. - Define the term ‘ionic bond’. - Define the term ‘cation’. - Define the term ‘anion’. Compounds made of a metal and a non-metal (salts) form ionic bonds. Sodium is a metal. It has 1 electron in its outer shell. It loses this electron to drop down to a full outer shell. It now has one less electron than protons, so is given the charge +, and we write it as Na+. Non-metals have 5 to 7 electrons in their outer shell. It requires less energy for non-metal atoms to take 1-2 electrons to fill their outer shell, rather than losing 5-6. electrons to drop down to the next full shell. Nonmetals always form anions. Metals only have one or two electrons in their outer shell. It requires less energy for metal atoms to give up electrons and drop down to the next full electron shell, rather than trying to find 6-7 electrons to fill the outer most shell. Metals always form cations. Fluorine is a non-metal. It has 7 electrons in its outer shell. It gains one extra electron to achieve a full outer shell. It now has one more electron than proton, so is given the charge -, and we write it as F-. Metal Ions ! - Metals in Group 1 always form ions with a +1 charge. ! - Metals in Group II always form ions with a +2 charge. TASK: Copy this info into your workbook. ! - Metals in Group III always form ions with a +3 charge. ! - Metals in Group IV usually have a +2 charge, but can also have a +4 charge. Assume a +2 charge unless otherwise stated. ! - Transition metals have a variety of ionic charges, but most form ions with +2 charge. ! - Metals in Groups V and VI can also have charges that vary. ! - If a metal has more than one common ion, the charge it takes is shown with Roman numerals Copper (I) = Cu+ Copper (II) = Cu2+ Non-metal Ions TASK: Copy this info into your workbook. - Elements in Group VII (halogens) always form ions with a -1 charge. ! - Elements in Group VI always form ions with a -2 charge. ! - Elements in Group V always form a -3 charge. ! - Non-metal elements in Group IV (C and Si) may form -4 ions. ! - Elements in Group VIII (noble gases) either have full outer shells or are happy with eight electrons in their outer shells. They are extremely stable and do not form ions. An example: Formation of table salt (Sodium Chloride) ! If a sodium atom meets a chlorine atom, the sodium loses its single outer shell electron to form the sodium cation Na+. Because is now has one more proton than electrons, it is given the charge +. Chlorine takes the electron from sodium to become the anion Cl-. It now has a new name: chloride. Because is now has one more electron than protons, it is given the charge -. ! Both ions are stable, and are attracted to each other. They exist together as Na+Cl-... sodium chloride. ! The attraction between the positive and negative ions is called the ionic bond. TASK: Copy this diagram into your workbook TASK: Copy this diagram into your workbook - Which element forms the cation? - Which forms the anion? TASK: Complete the following table on this slide. - You can work in pairs to complete this task. - You can also use the “smell-o-mints” App to help. TASK: Complete the following activity on this slide. In order to write the correct formulae for ionic compounds, you need to know the charge on each ion involved. To help you learn these, complete the tables below. Ions with opposite charges are attracted to each other, with negative charged ions and positive charged ions combining to form a neutral compound. This creates the ionic bond that holds them together. ! Ionic compounds are not made of separate molecules. Instead, the ions gather in a regular arrangement called an ionic lattice. ! Ionic bonds are very strong - much stronger than covalent bonds. It take a lot of energy to break ionic bonds, so ionic compounds have high melting and boiling points. TASK: Answer the following questions in your workbook. - Define the term ‘ionic lattice’. - Which type of elements forms cations? - Which type of element forms anions? Ionic Bonding Task: complete the following activity. “Ions get together” in your workbook. You can cut and paste the jigsaw pieces, or you can draw them Polyatomic Ions ! Some covalent compounds have charges - they are made up of more than one type of atom and have a charge. They are called polyatomic ions. These ions have special names. TASK: define the term “polyatomic ion’ your workbook, and list 2 examples. Naming Ionic Compounds To name ionic compounds, simply follow these rules: ! 1. The positive ion (cation) is named first and the negative ion (anion) second. ! 2. A simple positive ion (cation) takes its name from its parent element. For example, Na+ is called sodium. ! 3. A simple negative ion (anion) is named by taking the first part of the elements name and adding the suffix -ide. Cl- was originally a chlorine atom, and is given the new name chloride. ! We do not use prefixes when naming ionic compounds, unlike when we name covalent compounds. ! For example, an ionic substance made of one magnesium cation and two fluorine anions would have the formula MgF2 and the magnesium fluoride. Writing Ionic Formulae When trying to determine the chemical formula of an ionic compound (i.e. the number of cations and anions needed to give a neutral charge), you can draw your jigsaw pieces... ! Or you can make it easier by “crossing over”... ! Ca2+ and Cl- ! ! Ca2+1Cl-2 CaCl2 Calcium chloride ! Cr3+ and O2- ! ! Cr3+2 O2- 3 Cr2O3 Chromium oxide Place the charge at the top of each element Cross over each charge as subscripts Remove charges at top of element for final formula Place the charge at the top of each element Cross over each charge as subscripts Remove charges at top of element for final formula When more than one polyatomic ion is required in a formula, brackets are used. ! In magnesium sulfate, MgSO4, only one sulfate ion is needed. ! Mg2+ SO42- ! MgSO4 In aluminium sulfate, Al2(SO4)3, three sulfate ions are required and so brackets are used. ! Al3+ SO42- ! Al2(SO4)3 TASK: Complete the following activity.You can fill in the table on this slide, but you may need to do your “cross-overs” in your workbook. Use the cross-over method to help write formulae for some ionic compounds. + Na + Cl + Na Cl 1 sodium ion joins with 1 chloride ion to form sodium chloride. The formula is NaCl. Na+ Cl- ! NaCl 2+ Mg + Cl + Cl Cl 2+ Mg Cl 1 magnesium ion joins with 2 chlorine ions to form magnesium chloride. The formula is MgCl2. Mg2+ Cl- ! MgCl2 TASK: Answer the following questions in your workbook. Write the formulas for the following ionic compounds: 1. Sodium bromide 2. Magnesium sulfide 3. Calcium fluoride 4. Lithium nitride 5. Aluminium carbide 6. Magnesium hydroxide 7. Lithium nitrate 8. Sodium sulfate TASK: Answer the following questions in your workbook. Write the chemical names for the following covalent compounds: 1. FeCl2 2. Cu(NO3)2 3. NH4OH 4. MgO 5. KBr 6. ZnCl2 7. Li3N 8. SrS Extension Questions Level 1 - Hard [knowledge extension activity] Level 2 - Challenging [application question, linked to Year 11 Chemistry syllabus] If you are considering studying Physics or Chemistry next year, or just want a challenge, attempt the following activities. ! And if you need help, make sure you ask Ms Belshaw. In today’s keynote, Physics and Chemistry students should attempt to complete BOTH LEVELS. Level 1: More ionic compounds TASK: Complete the following table. ! Level 2: Valency TASK: Use information here to answer the question on the following slide. Write your answers in your workbook The number of electrons an atom needs to gain or lose to form a stable outer shell of electrons is called its valency or combining power. An atom with a stable outer shell of electrons has a valency of zero. If an atom needs to gain or lose just one electron, it has a valency of 1.If an atom needs to gain or lose 2 electrons, is has a valency of 2, and so on ... The valency of an ion is the same size of its charge. For example, the oxide ion (O2-) has a charge of 2- and a valency of 2. ! Some elements can form different ions, so can have more than once valency. Iron, for example, forms Fe2+ and Fe3+. Roman numerals after the name, for example iron (II) and ion (III) indicate the valency. Using the internet, fill in the table below, identifying the electron configuration and valency for the first 20 elements in the periodic table. Element Symbol H He Li Be Be C N O F Ne Na Mg Al Si P S Cl Ar K Ca Electron Configuration Valency






