Polar Molecules
advertisement

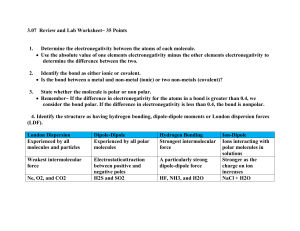
Polar Molecules Nelson Chemistry 12 – Section 4.4 (pages 251-256) Part A Read Section 4.4 of your textbook. Answer the following questions as you read: 1. What is a polar molecule? - A polar molecule is one in which the charge is not distributed symmetrically among the atoms making up the molecule 2. What is the difference between a polar molecule and a charged molecule (or ion)? - Polar molecules do not have a net charge, just a unsymmetrical distribution of charge 3. Define electronegativity. - The attractive force an atom’s nucleus exerts on electrons. When atoms bond, the atom with a greater attraction for electrons will have the pair of electrons in its orbital for a greater amount of time (shared unequally) 4. Describe the trends of electronegativity (increasing/decreasing) across periods (horizontal) and groups (vertical) on the periodic table. - Electronegativity increases when moving to the right and up the periodic table. Therefore, fluorine is the most electronegative (top right) and francium is the least electronegative (bottom left). 5. Explain how to determine whether a chemical bond is non-polar covalent, polar covalent, or ionic. - Depends on the difference in electronegativity (i.e., the difference in attraction for the pair of electrons in a bond). The greater the difference, the more polar. - A polar bond is formed when two different kinds of atoms form a bond; these atoms have differing electronegativities, and therefore, share the bonded pair of electrons unequally – they spend more of their time closer to the more electronegative atom. - A nonpolar bond forms between two atoms that share electrons equally; these atoms most have the same (or very close to the same) electronegativity. - Ionic bonds form when the difference in electronegativity exceeds 1.7. This generally only occurs between metals and non-metals. 6. Explain the meaning of this statement – “The difference between a polar covalent bond and an ionic bond is in shades of grey, rather than black or white.” - The type of bond formed, whether it be nonpolar, polar, or ionic, occurs on a spectrum. There are no cut and dry terms to define a polar bond from an ionic bond; however, it is generally agreed upon that at an electronegativity difference of 1.7, bonds are more likely to be ionic. 7. For each of the following, label the electronegativities. Label any partial charges and classify as ionic, polar covalent, or non-polar covalent. Electronegativity of atoms can be found on the periodic table at the back of your text. a. H – Cl b. C – H c. N – O d. I – Br e. Mg – S f. P – H 8. Explain the difference between a polar bond and a polar molecule. - Polar bonds are the unequally sharing of electrons in a shared bond (due to differences in electronegativity) - Polar bonds, however, can cancel out depending on the geometry of the molecule. If bond dipoles (the vector pull on electrons due to unequal sharing) can cancel out, the molecule will have polar bonds, but will be a nonpolar molecule. If the geometry or bonding atoms do not allow the bond dipoles to cancel, the molecule will be polar. 9. Explain why carbon tetrafluoride (CF4) is a non-polar molecule, even though it possesses polar bonds. - CF4 is tetrahedral in shape. Each C – F bond is polar, with the F-atom having a higher electronegativity (and a resulting slightly negative charge). - However, CF4 is a nonpolar molecule because it is a symmetrical molecule and the bond dipoles will cancel out 10. Predict whether the following molecules are polar or non-polar. Include the VSEPR diagram of the molecule and bond dipoles to make an accurate prediction. a. Boron trifluoride, BF3 b. Oxygen difluoride, OF2 c. Carbon tetraiodide, CI4 d. Phosphorous trichloride, PCl3 e. Dichlorofluoroethane, CHFCl2 f. Ethene, C2H4 g. chloroethane, C2H5Cl h. Methylamine, CH3NH2 i. Ethanol, C2H5OH j. Diboron tetrafluoride, B2F4



![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)