CHEM 109A CLAS Alkenes and Reactions of Alkenes
advertisement

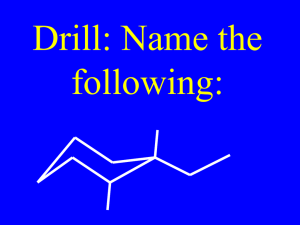
CHEM 109A CLAS Alkenes and Reactions of Alkenes - KEY 1. Predict the product(s) for each of the following reactions. If there is more than one product, predict the relative yield (a.k.a. label the major product). H a. Br 2-butene H 2-butene Br- Br hydrobromic acid H Br H H b. Cl cyclohexene H Cl Cl Cl cyclohexene H H H c. trans-3-methyl-pent-2-ene H H H I I trans-3-methyl-pent-2-ene I- H d. I Cl 1-methylcyclopentene H H H Cl Cl- Cl 1-methylcyclopentene H 2O e. 2-butene H 2SO 4 Page 1 of 6 CHEM 109A CLAS Alkenes and Reactions of Alkenes - KEY H H O H 2O 2-butene + H H O H HSO4- H 2SO 4 H H O H H H O+ O H H H O+ H H H CH 3OH f. H 2SO 4 3-methyl-1-butene H O+ H H H 3-methyl-1-butene H HSO4- H O H H H O+ H O O H H H O+ H H CH3CH 2OH g. cyclopentene H 2SO 4 Page 2 of 6 CHEM 109A CLAS Alkenes and Reactions of Alkenes - KEY H O O+ CH 3CH2OH H H H 2SO 4 cyclopentene H HSO4- O+ H O H O O+ H H H H 2. Fill in the missing information in the following reactions Cl HCl a. 3-chlorohexane 2-hexene or 3-hexene Br b. 2-bromo-2-methylbutane HBr or 2-methyl-but-2-ene 2-methyl-1-butene O c. propoxycyclohexane cyclohexene HO propanol H2SO 4 OH d. 2-pentene 2-pentanol HO H 2O H 2SO 4 also 3-pentanol Page 3 of 6 CHEM 109A CLAS Alkenes and Reactions of Alkenes - KEY O e. 1-isopropoxy-2-methylbutane OH 1-propene H 2SO 4 Additional Information: Alkenes (CnH2n, if cyclic CnH2n-2) – hydrocarbons that contain a carbon-carbon double bond. Nomenclature Alkenes (CnH2n): -ene 1. Determine the # of C atoms in the longest continuous chain (parent chain) that CONTAINS the double bond – Watch for branches! a. Use a prefix for # of double bonds (EX. diene for 2) b. A # is not needed to denote position of double bond in ring, b/c ring is numbered so that double bond is on C #1. 2. Number the chain in the direction that gives the double bond the lowest #. a. If numbering in both directions gives the same number to the double bond, number in the direction that gives the lowest number to the alphabetically first substituent. 3. Substituents are listed in alphabetical order. O EX. 2-hexene 2,3-dimethyl-2-pentene 4-hexoxy-1-butene 2-ethylcyclopentene Structural Details Vinyl (sp2 Cs in double bond) and allyl (sp3 Cs adjacent to double bond) Cs are in same plane, double bond is above and below the plane. Get cis-trans/Z-E (a.k.a. geometric) isomers if vinyl Cs have different groups, and they are separable. Cis-trans do NOT interconvert b/c to rotate, the π bond would have to break (∆E C-C π = 62 kcal/mol vs. ∆E C-C σ = 2.9 kcal/mol). Reactions of Alkenes – See also Summary of Reactions of Alkenes Page 4 of 6 CHEM 109A CLAS Alkenes and Reactions of Alkenes - KEY Electrophilic Addition Rxns In general: π e-s of double bond attracted to electrophile (alkene is a Nuc) & get addition of electrophile to 1 vinyl C and Nuc adds to the other vinyl C. Can be used to synthesize alkyl halides (EX above), alcohols, ethers, epoxides and alkanes. Addition of Hydrogen Halide (HF, HCl, HBr, HI) Fig 4.1 shows hyperconjugation Electrophile (proton) adds to least substituted (greatest # of Hs) vinyl C to form the more stable carbocation and Nuc adds to carbocation → regioselective (one constitutional isomer is preferred) → a.k.a. Markovnikov addition. H H Br Br- hydrobromic acid 2,3-dimethyl-2-butene 3o carbocation intermediate H Br Addition of Water (acid-catalyzed) – a.k.a. hydration Acid (often H2SO4) required b/c otherwise there is no electrophile. Also need unreactive solvent to dissolve the alkene (ex. DMSO). Markovnikov add H and –OH. O O H O H water H HO S O+ OH H -O H H O+ H O H H 2,3-dimethyl-2-butene H 3o carbocation intermediate O H H H OH O O sulfuric acid O+ S H H H H H O + HO H H Page 5 of 6 CHEM 109A CLAS Alkenes and Reactions of Alkenes - KEY Addition of Alcohol (acid-catalyzed) – forms an ether Acid (often H2SO4) required b/c otherwise there is no electrophile. Also need unreactive solvent to dissolve the alkene (ex. DMSO). Markovnikov add H and –OR. O O H O HO H S O+ OH -O S OH H O O sulfuric acid H H O+ O H H 2,3-dimethyl-2-butene 3o carbocation intermediate H H O H H H O + O+ H O Page 6 of 6