Alkanes and Alkenes 2002 Q17
advertisement

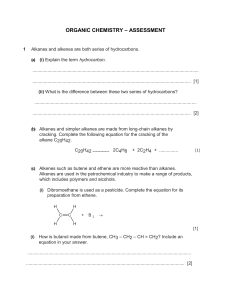
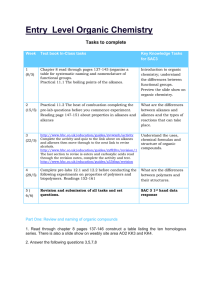
Friday pHunday ALKANES AND ALKENES (HSC 2002 Q17, 3 marks) Explain why alkanes and their corresponding alkenes have similar physical properties, but very different chemical properties (3 marks) Explain relate cause and effect; make the relationships between things evident; provide why and / or how. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… HSC 2002 - Q17 Physical Properties: Cause: Both alkanes and alkenes are non polar hydrocarbon molecules. Effect: Their dipoles are arranged symmetrically so they cancel each other out (the dipoles do not reinforce each other). As a result, their strongest intermolecular forces are dispersion forces. Physical properties including melting and boiling point and solubility are dictated by these forces. So physical properties in alkanes and alkenes are similar. Chemical Properties. Cause: Alkanes have single carbon-carbon covalent bonds and are therefore saturated. Alkenes have at lease one carbon-carbon double bond and are unsaturated. Effect: Alkanes are therefore less reactive chemically than alkenes.