Bohr-Rutherford Diagrams Worksheet: Atomic Structure
Name: ______________________________
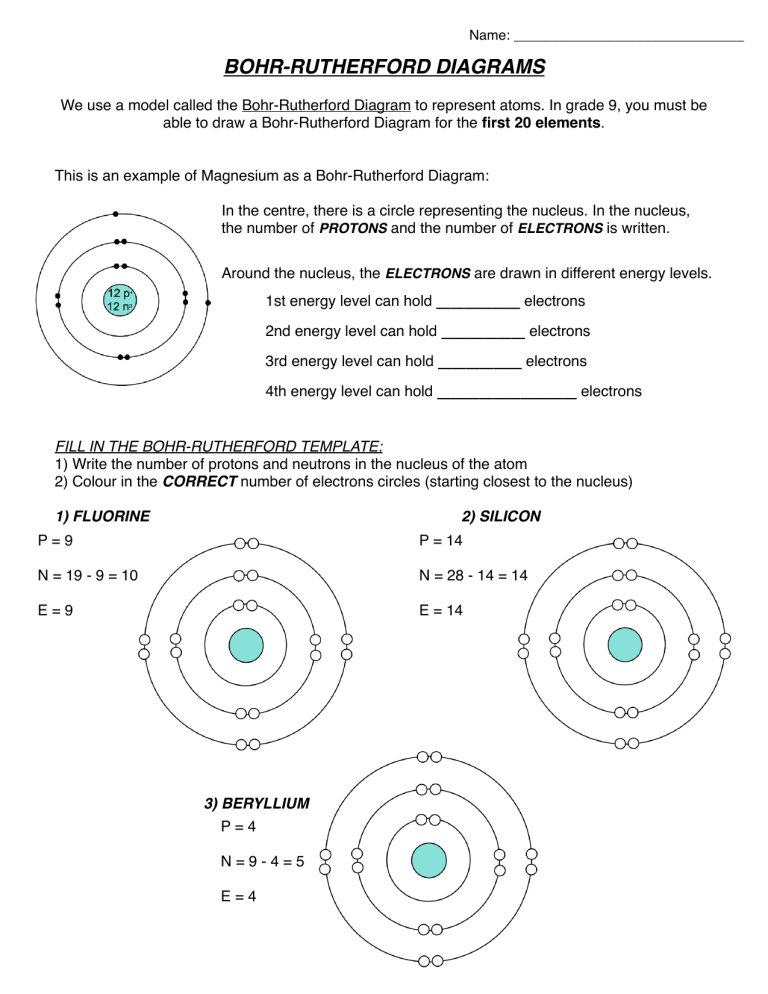
BOHR-RUTHERFORD DIAGRAMS
We use a model called the Bohr-Rutherford Diagram to represent atoms. In grade 9, you must be able to draw a Bohr-Rutherford Diagram for the first 20 elements .
This is an example of Magnesium as a Bohr-Rutherford Diagram:
In the centre, there is a circle representing the nucleus. In the nucleus, the number of PROTONS and the number of ELECTRONS is written.
Around the nucleus, the ELECTRONS are drawn in different energy levels.
1st energy level can hold
______
electrons
2nd energy level can hold
______
electrons
3rd energy level can hold
______
electrons
4th energy level can hold
__________
electrons
FILL IN THE BOHR-RUTHERFORD TEMPLATE:
1) Write the number of protons and neutrons in the nucleus of the atom
2) Colour in the CORRECT number of electrons circles (starting closest to the nucleus)
1) FLUORINE
P = 9 P = 14
2) SILICON
N = 28 - 14 = 14 N = 19 - 9 = 10
E = 9 E = 14
3) BERYLLIUM
P = 4
N = 9 - 4 = 5
E = 4
HOW TO DRAW A BOHR-RUTHERFORD DIAGRAM (from scratch)
1. Determine the number of protons, neutrons and electrons in the atom.
ELEMENT = Boron PROTONS =
NEUTRONS =
ELECTRONS =
2.
Draw a circle to represent the nucleus.
In this circle, write the number of protons (
____
) and the number of neutrons (
____
)
3. Draw a circle around the nucleus to represent the first energy level. Fill this energy level with the correct number of electrons (max. = 2). If necessary, add a second energy level, and fill it as needed (max. = 8).
If necessary, add a third energy level (max. = 8) and a fourth energy level (max. = 18). STOP ADDING
ELECTRONS when you reach the number occurring in the atom. For example, Boron has 5 electrons.
ANOTHER EXAMPLE: Chlorine
ONE MORE EXAMPLE: Helium
Draw Bohr-Rutherford Diagrams for the following atoms:
HYDROGEN OXYGEN
# P = # N = # E =
SULFUR
# P = # N = # E =
SODIUM
# P = # N = # E =
CALCIUM
# P = # N = # E =
NEON
# P = # N = # E = # P = # N = # E =








