Bohr-Rutherford diagrams for atoms
advertisement

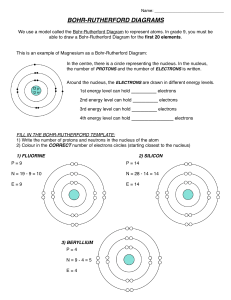
Name: Bohr-Rutherford diagrams for atoms Bohr stated that… • Electrons move around the nucleus in planets around the Sun. • The for an element is the period number. • The maximum number of electrons in an orbital is . , like Bohr-Rutherford Diagram • Bohr-Rutherford diagrams represent the arrangement of in an element. • In these diagrams, the number of protons and neutrons are written in the the nucleus of the atom. • Circles are drawn around the nucleus to represent orbitals, and electrons are shown in these orbitals Example of a Bohr-Rutherford diagram: Lithium (Li) Atomic Number = Atomic Mass = #protons = # electrons = # neutrons = 3p 4n Rules for adding electrons to orbitals • The first orbital can hold up to electrons. • The second and third orbital can hold up to electrons. • When filling orbitals with electrons, place the first 4 electrons in a before pairing. Example of a Bohr-Rutherford diagram: Aluminum (Al) Atomic Number = Atomic Mass = #protons = # electrons = # neutrons = 13p 14n Bohr-Rutherford diagrams of the first twenty elements: • These electrons in the outer orbital are called 3p 4n • Draw the Bohr-Rutherford diagrams of the first TWENTY elements on the chart provided.