Bohr-Rutherford Diagrams and the Periodic Table
advertisement

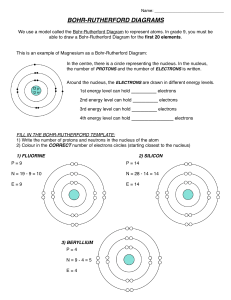
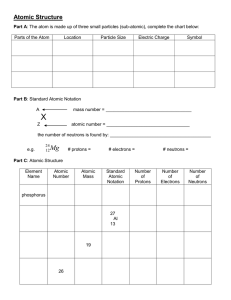
Bohr-Rutherford Diagrams and the Periodic Table Steps to drawing Bohr-Rutherford diagrams 1. Write the symbol of the element in Standard Atomic Notation. - Write the Symbol of the element - Write the Mass Number, the amount of particles in the nucleus, as a superscript to the right of the symbol. - Write the Atomic Number, the number of protons in the nucleus, a a subscript to the right of the symbol 2. Infer the number of neutrons. - Remember mass number = # protons + # neutrons, so the # of neutrons = Mass number – Atomic Number 3. Find the number of Electrons - Remember in neutral atoms the # of electrons = # of protons 4. Drawing the nucleus - Draw a circle, in the circle write the number of protons and neutrons 5. Drawing electron configuration - Draw a circle around your nucleus - Place an x (or a dot) to represent each of the electrons in that shell (the first shell can hold only 2 electrons. - Draw another circle, starting from the top and then a quarter way around the circle draw in the electrons one at a time until the shell is full (8 electrons). Continue with more shells as needed Complete the following table Name element Symbol Atomic # 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Mass Number # P #E #N In the table below draw a Bohr-Rutherford diagram for the first 20 of the elements on the periodic table. 1 2 The Periodic Table of Elements And Bohr-Rutherford Diagrams 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 1. What do you notice about the # of electrons as you move along a row? 2. Describe the reactivity between the families of elements? 3. What do you notice about the # of shells as you move from one period to another 4. Describe what happens to reactivity as you move down the metals families? Up the non-metals families?