Hydrates(and an introduction to Stoichiometry!) Introduction Purpose
advertisement

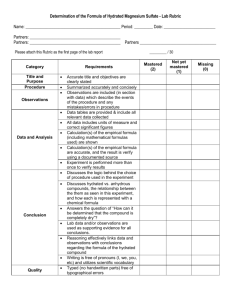
Name: Hydrates (and an introduction to Stoichiometry!) Introduction This lab will serve as an introduction to stoichiometry. Specifically, you will be attempting to do two things: predict an amount of product from a given amount of reactant, and determine the formula of a certain hydrated compound whose number of "waters of hydration" is unknown. A little background information concerning hydrated compounds: Often, when solid salts are obtained by evaporating the water from the solution in which they are dissolved, some water molecules remain attached to the solid, crystalline salt. For example, if you have a solution of copper(II) sulfate, CuSO4, and you slowly evaporate the water from the solution, eventually you will end up with a blue crystalline solid which has the formula CuSO4.5H2O. The ".5H2O" is read as "pentahydrate", and means that each copper sulfate molecule is weakly attached to 5 molecules of water. The weak attachment, a weak bond, can easily be broken by applying heat. Hydrated compounds are crystalline, whereas the anhydrous (“without water”) form of the compound does not form crystals, and usually occurs as a powder. Purpose Predict the amount of anhydrous compound which will remain after a hydrated compound is heated, and to experimentally determine the formula of an “unknown” hydrated salt. General Procedure PART 1: The first salt you use will be one you already know: copper(II) sulfate pentahydrate. Obtain a small amount (a small scoop-full) and determine its mass precisely. Predict, using stoichiometry, the amount of anhydrous CuSO4 which will remain after the water is removed entirely. Write and perform your own experimental procedure, and compare your result with your predicted amount, and determine your percent yield. Do not throw the anhydrous copper(II) sulfate away – place it in the container in the front of the lab. PART 2: Obtain one of the compounds which has an unknown number of waters of hydration. Determine the correct formula for the hydrated form of the compound. Note: Be sure to record all observations. You should start by making a complete, neat data table, which includes spaces for all the measurements that you will need to eventually record. Questions for Discussion and Research hydration Bunsen burner the compounds encountered in this lab exothermic/endothermic constant massing