Water of crystallisation
advertisement

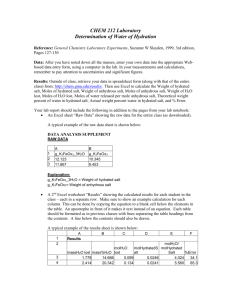
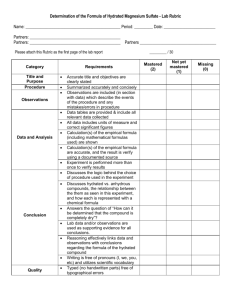
Water of crystallisation L.O.: Explain the terms anhydrous, hydrated and water of crystallisation. Calculate the formula of a hydrated salt. Workshop today at 1:45pm: Winifred Henry Anna Keverne Esther Adeduntan Copper sulfate • Copper sulfate comes in a hydrated form (copper sulfate pentahydrate) and a anhydrous form http://uk.youtube.com/watch?v=kp7yaxlYk08 Anhydrous substances • Many substances will absorb water from the atmosphere, like NaCl, these are called hygroscopic substances • An anhydrous compound is one which has no water molecules inside of it • To make an anhydrous substance you can either heat it to evaporate off the water, or you can dry it in a drying cabinet Writing out the formula for hydrated compounds • There are two ways in which a formula can be written if it is a hydrated compound: 1) Empirical formula, Which you know and love OR 2) Dot formula, So copper sulfate pentahydrate becomes Cu SO3 • 5H2O NOTE: This dot is very important!!!!! Hydrated substances and water of crystallisation • Some substances can trap water molecules inside of them. • The water contained is called the water of crystallisation Finding the formula of hydrated copper(II) sulfate Note: see worked example in page 27 FeSO4·xH2O Exp 1 Mass of crucible Mass of crucible + hydrated salt Mass of hydrated salt Mass of anhydrous salt Mass of water lost Exp 2 FeSO4·xH2O Exp 1 Mass of anhydrous salt Mass of water lost Exp 2 Mean mass FeSO4·xH2O Mass of anhydrous salt Mean mass 1.51 g Mass of water lost 0.54 g 1) Calculate amount in moles of anhydrous salt n (FeSO4) = 1.51/ 151.8 = 0.01 2) Calculate the amount in moles of water. n (H2O) = 0.54/18 = 0.03 3) Calculate molar ratio FeSO4 : H2O = 0.01: 0.03 4) Divide be the smallest number 0.01: 0.03 = 1:3 Salt CaCl2•2H2O ZnCl2•6H20 Ba(OH)2•8H2O Cu SO3 • 5H2O YSO3•6H20 Na Z XSO4 Ions present Hydrated salt Mass of anhydrous salt Water lost XCl•2H2O 5.86 g 9g XSO4•3H20 40.53 g 16.2 Identity of salt Other examples of hydrated compounds: • Cobalt chloride hexahydrate • Sodium sulfate decahydrate Write out the formula for these compounds using the “dot formula” notation Convert these into the Dot Formula 1. 2. 3. 4. Calcium chloride dihydrate Calcium chloride hexahydrate Zinc chloride hexahydrate Barium hydroxide octahydrate Answers 1. 2. 3. 4. Calcium chloride dihydrate = CaCl2•2H2O Calcium chloride hexahydrate = CaCl2•6H2O Zinc chloride hexahydrate = ZnCl2•6H20 Barium hydroxide octahydrate = Ba(OH)2•8H2O Write these out in Dot formula 1. Hydrated Cobalt chloride CoCl2H12O6 2. Hydrated Calcium carbonate CaCH6O6 3. Hydrated Magnesium sulphate MgSH12O10