HYDRATED CRYSTALS LAB
advertisement

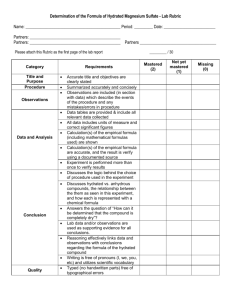
HYDRATED CRYSTALS LAB CONCEPT Some crystalline salts retain water as a component of their chemical formulas. This water is molecularly bonded to the crystalline salt molecule. An example of a hydrated compound is Sodium carbonate decahydrate, Na2CO3•10H2O. Notice that in this compound there is a 1:10 ratio between the Na2CO3 and the H2O. This ratio will always be a whole number. Heating a hydrated compound will remove the water and the salt will become anhydrous, meaning “without water”. This process is analogous to placing clothing in the dryer in order to separate water from clothing. OBJECTIVE • Use mass measurements to determine an empirical formula, or ratio, between water and anhydrous compound for: Magnesium sulfate - MgSO4• XH2O and Copper (II) Sulfate - CuSO4 •XH2O. EQUIPMENT Metal crucible and cover Clay triangle Ring stand and ring Bunsen burner PROCEDURE 1. Measure the mass of a clean crucible and cover. Record in Data Table. 2. Put one full measuring spoon (plastic) of Magnesium sulfate in the metal crucible, cover, and measure the total mass. Record in Data Table. 3. Heat for 10 minutes. Let cool for 15 minutes and remove from the ring stand. 4. When no longer warm to the touch (room temperature) measure mass of crucible, cover and sample. Record in Data Table. 5. Complete necessary calculations to determine empirical formula and percent error. Name: ______________________________________ Period: ________ Date: ______________ HYDRATED CRYSTALS LAB DATA TABLE Magnesium sulfate MgSO4•XH2O Copper II Sulfate CuSO4 •XH2O Crucible, cover & sample 39.47 g g Crucible and cover 35.44 g g g g hydrated sample Crucible, cover & heated sample 37.84 g g Crucible and cover 35.44 g g Anhydrous sample g g H2O in sample g g Moles of H2O in sample mol mol Moles of anhydrous sample mol mol Calculations: 1. Calculate the moles of H2O in each sample (mass of H20 from data table / molar mass H20) enter in DATA TABLE above. Copper II Sulfate 2. Magnesium Sulfate Calculate the moles of each anhydrous compound (compound without water attached) and enter in DATA TABLE above. Copper II Sulfate Magnesium Sulfate 3. Derive an empirical formula (ratio between H20 and anhydrous compound) for each hydrated samples from your lab results. Use the number of moles of each calculated in steps 1 and 2. This ratio becomes the coefficients in the compound: __CuSO4•__H20 or __MgSO4•__H2O Copper II Sulfate 4. Calculate the percent of water in a perfect sample (Use the formulas: CuSO4•5H2O and MgSO4•7H2O) of each hydrated compound. Copper II Sulfate 5. Magnesium Sulfate Calculate the percent of water in each hydrated compound from your lab results. (Use the formula you calculated in step 3 based on your mole ratio) Copper II Sulfate 6. Magnesium Sulfate Magnesium Sulfate Calculate your percent of error based on results of 4 and 5. (Use your final % answers and plug in to % error formula: ( | Actual – Experimental | / Actual ) x 100 Copper II Sulfate Magnesium Sulfate