File

LAB – Hydrate Crystals
OBJECTIVE:
In this experiment, you will:
Heat a specific amount of hydrated CuSO
Find the percent water in the hydrate
Find the empirical formula of the hydrate
4
to remove the water.
BACKGROUND:
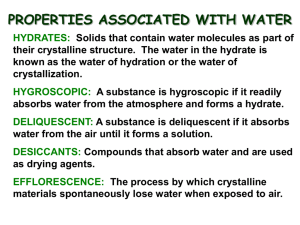
Many compounds are formed in reactions that take place in aqueous (water) solutions. The water is then evaporated to obtain the crystalline compound. In some cases, water molecules are weakly attracted to the ions or molecules that make up the compound and are retained within the crystal structure. Crystalline compounds that retain water during evaporation are referred to as being hydrated or are said to contain water of hydration. The ratio of moles of water to moles of compound is a small whole number.
The ratio of moles of water to moles of compound can be determined experimentally in most cases by heating to remove the water. The compound with the water removed is anhydrous. In this experiment, you will determine the formula for hydrated copper (II) sulfate. The formula is determined by comparing the mass of the hydrated and anhydrous forms of the compound.
MATERIALS:
Large test tube
Balance (0.01 g precision)
SAFETY FIRST:
Test tube clamp
Copper (II) sulfate
Striker
Bunsen burner
Wear safety goggles and lab apron at all times. Do NOT point the open end of the test tube towards yourself or your lab partners.
PROCEDURE:
1.
Measure and record the mass of clean, empty and dry test tube to the nearest 0.01 g.
2.
Add between 2.50 g and 3.00 grams of CuSO
4
to the test tube. Record the mass of the copper (II) sulfate and the test tube in the data table.
3.
Heat the hydrate for about 15-20 minutes, or until absolutely no steam is seen and no moisture is present inside the mouth of the test tube (refer to figure 1).
CAUTION: Do NOT point the open end of the test tube towards yourself or your lab partners.
4.
Let the anhydrous copper (II) sulfate cool completely.
5.
Measure and record the mass of the anhydrous copper (II) sulfate.
6.
Empty the anhydrous copper (II) sulfate into the beaker on the teacher’s desk after steps 1-5.
7.
Complete the data table and show calculations called for below the data table.
Figure 1
Name ____________________________________________________ Date ________________________ Period ________
Group Member Names _________________________________________________________________________________
LAB – Hydrated Crystals (Data Sheet)
OBSERVATIONS:
DATA TABLE 1: Mass Measurements
Item
Test tube
Test tube and hydrated CuSO
4
Hydrated CuSO
4
Test tube and anhydrous CuSO
4
Anhydrous CuSO
4
H
2
O in the hydrate
Mass (g)
ANALYSIS AND CONCLUSIONS
Show ALL work with units and round all answers to the correct number of significant figures. All answers must have a
UNIT and FORMULA.
1.
Calculate the moles of anhydrous copper (II) sulfate.
2.
Calculate the moles of water in the hydrate.
3.
Calculate the percent water in the hydrate.
#1 Answer:
#2 Answer:
#3 Answer:
4.
Determine the formula for the hydrated CuSO
4
. Because these are experimental results you may not get a whole number coefficient. Therefore, show your answer with the correct significant figures before writing your formula and then round it to a whole number. You should have whole numbers in your final answer.
#4 Answer:
5.
The theoretical formula is CuSO
4
•5H
2
O. Give two possible sources of error that explain your result for this lab. a.
_______________________________________________________________________________________
_______________________________________________________________________________________
_______________________________________________________________________________________ b.
_______________________________________________________________________________________
_______________________________________________________________________________________
_______________________________________________________________________________________