Acetic Anhydride: Properties, Reactions & Uses
advertisement

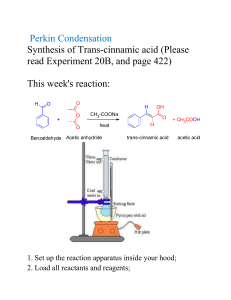
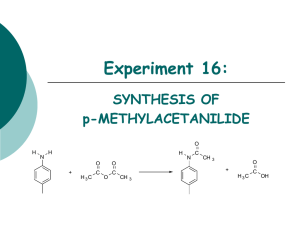
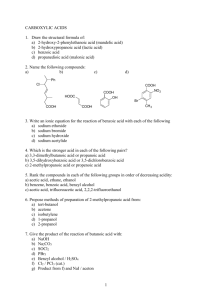
PRODUCT DESCRIPTION 1 of 3 Acetic Anhydride l CH3–C ll CH3–C O l MW = 102.09 O ll (Ethanoic Anhydride, Acetyl Oxide, Acetyl Ether, Acetic Acid Anhydride) O Acetic Acid, Anhydride, Chemical Abstracts Registry Number 108-24-7 Wiswesser Line Formula Chemical Notation 1V0V1 Acetic Anhydride is a clear, colorless liquid with a very pungent, penetrating, vinegar-like odor. It is soluble in ether, chloroform and benzene. It reacts with alcohols. Acetic anhydride is not very soluble in water, but reacts with it to form acetic acid. Acetic Anhydride is widely employed industrially for its acetylating and dehydrating properties. A major use for it is the acetylation of cellulose to produce acetate fibers, plastics, coatings and films. It is especially valuable for the direct esterification of alcohols where acetic acid cannot be used. Another large use for Acetic Anhydride is in the manufacture of acetylsalicylic acid (aspirin), acetophenacetin, aceto-p-aminophenol, cortisone, acetanilide, theophylline, sulfa drugs, certain vitamins and hormones, and many other pharmaceuticals and pharmaceutical intermediates. Acetic Anhydride is also used to produce acetyl ricinoleates, triacetin, acetyl tributyl citrate, and other plasticizers. Another chemical use is in the manufacture of acetyl peroxide. Acetic Anhydride is also used as an intermediate in the manufacture of explosives, weed killers, and in the chemical treatment of paper and textiles. Animal and vegetable oils are acetylated with Acetic Anhydride to change their solubility characteristics. The reactions of Acetic Anhydride are those of a typical acid anhydride. PRODUCT DESCRIPTION 2 of 3 Acetic Anhydride Chemical Reactions 1. Acetylation CH3COONa C6H4CH3NH2 + (CH3CO)2O → C6H4CH3NHCOCH3 + CH+COOH 2. Conversion into acids and acid derivatives (a) Hydrolysis into acetic acid (CH3CO)2O + H2O →2CH3COOH (b) Ammonolysis into acetamide (CH3CO)2O + 2NH3 →CH3CONH2 + CH3COONH4 (c) Alcoholysis into esters (CH3CO)2O + CH3OH →CH3COOCH3 + CH3COOH 3. Formation of ketones by Friedel-Crafts acylation AICI3 → (CH3CO)2O + ArH Lewis Acid CH2COAr + CH3COOH 4. Condensation Reactions (Perkin) CH3COONa C6H5CHO + (CH3CO)2O C6H5CH=CHCOOCH3 + CH3COOH → ∆ PRODUCT DESCRIPTION 3 of 3 Acetic Anhydride Physical Properties Autoignition Temperature °C Boiling Point at 760 mm Hg, °C Boiling Point at 760 mm Hg, °F Coefficient of Thermal Expansion per °C at 20°C Critical Pressure, atmospheres Critical Temperature, °C Dielectric Constant, at 20°C Evaporation Rate (BuAc = 1) Flammable Limits (lower limit, vol %) (upper limit, vol%) Flash Point Tag Open Cup, °F Tag Closed Cup, °F Freezing Point, °C Heat of Combustion, kcal/mole This information is based on our present state of knowledge and is intended to provide general notes on our products and their uses. It should therefore not be construed as guaranteeing specific properties of the products described or their suitability for a particular application. Any existing industrial property rights must be observed. The quality of our products is guaranteed under our General Conditions of Sale. PB-042-3 05/00 9072 392 139.6 283.3 1.12 x 10 –3 46 296 20.5 0.46 2.7 10.3 148 121 -73.1 431.9 Heat of Vaporization, cal/gm at 15°C Molecular Weight (formula) Refractive Index n20 D Solubility at 20°C, wt%, in water Solubility in Alcohol Solubility in Carbon Tetrachloride Solubility in Chloroform Solubility in Ether Specific Gravity, 20/20°C Specific Tension in Air at 20°C, dynes/cm Vapor Density (air = 1) Vapor Pressure, 20°C, mm Hg Viscosity at 20°C, centipoise Weight, pounds per gallon at 20°C (68°F) 121 102.09 1.3901 12 Decomposes Infinite Infinite Soluble Infinite 1.0828 32.7 4.0 4.0 0.901 9.01 Dallas: 1601 West LBJ Freeway Dallas, Texas 75234-6034 Tel.: 972 443-4000 Frankfurt: Lurgialle 14 D-60439 Frankfurt am Main Tel.: 0049/69-305-13300