CARBOXYLIC ACIDS
advertisement

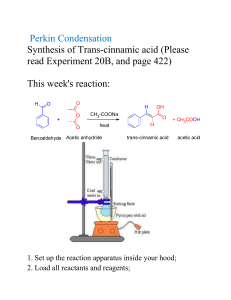
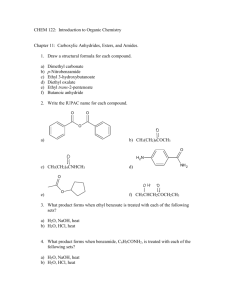
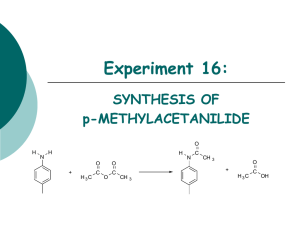
CARBOXYLIC ACIDS 1. Draw the structural formula of: a) 2-hydroxy-2-phenylethanoic acid (mandelic acid) b) 2-hydroxypropanoic acid (lactic acid) c) benzoic acid d) propanedioic acid (malonic acid) 2. Name the following compounds: a) b) c) d) Ph Cl COOH OH HOOC COOH COOH NO 2 Br CH 3 COOH 3. Write an ionic equation for the reaction of benzoic acid with each of the following a) sodium ethoxide b) sodium bromide c) sodium hydroxide d) sodium acetylide 4. Which is the stronger acid in each of the following pairs? a) 3,3-dimethylbutanoic acid or propanoic acid b) 3,5-dihydroxybenzoic acid or 3,5-dichlorobenzoic acid c) 2-methylpropanoic acid or propenoic acid 5. Rank the compounds in each of the following groups in order of decreasing acidity: a) acetic acid, ethane, ethanol b) benzene, benzoic acid, benzyl alcohol c) acetic acid, trifluoroacetic acid, 2,2,2-trifluoroethanol 6. Propose methods of preparation of 2-methylpropanoic acid from: a) tert-butanol b) acetone c) isobutylene d) 1-propanol e) 2-propanol 7. Give the product of the reaction of butanoic acid with: a) NaOH b) Na2CO3 c) SOCl2 d) PBr3 e) Benzyl alcohol / H2SO4 f) Cl2 / PCl3 (cat.) g) Product from f) and NaI / aceton 1 h) i) j) k) Br2 / PBr3 (cat.) Product from h) and NH3 (excess) 1) LiAlH4 2) H3O+ Ph-Mg-Br 8. Show how butanoic acid may be converted to each of the following: a) 1-butanol f) 4-octanone b) butanal g) 2-bromobutanoic acid c) 1-chlorobutane h) 2-butenoic acid d) butanoyl chloride i) n-butyl butylate e) phenyl propyl ketone j) sodium butylate 9. Show by a series of equations, using any necessary organic and inorganic reagents, how acetic acid can be converted to each of the following compounds: a) aminoacetic acid e) ethyl acetate b) methoxyacetic acid f) ethyl iodoacetate c) bromoacetic acid g) acetic anhydride d) propanedioic acid (malonic acid) h) acetyl fluoride 10. Predict the product: a) acetic anhydride + MeOH / H+ b) benzoic anhydride + NH3 c) acetyl chloride + bromobenzene / AlCl3 d) benzoyl chloride + sodium benzoate e) p-chlorobenzoyl chloride + NH3 (excess) f) succinic anhydride + H2O g) succinic anhydride + 2 NaOH h) succinic anhydride + NH4OH i) butyrolacton + NaOH j) N-methylpyrrolidone + NaOH k) Acetanilide + HCl / H2O (heat) 11. Using ethanol as the ultimate source of all the carbon atoms, along with any necessary inorganic reagents, show how you could prepare each of the following: a) acetyl chloride b) acetic anhydride c) ethyl acetate d) ethyl bromoacetate e) acetamide f) 2-hydroxypropanoic acid 12. Using toluene as the ultimate source of all the carbon atoms, along with any necessary inorganic compounds, show how you could prepare each of the following: a) benzoyl chloride b) benzoic anhydride c) benzyl benzoate d) benzamide e) benzonitrile f) benzyl cyanide g) aniline 2 13. A certain carboxylic acid (C14H26O2), which can be isolated from whale blubber or sardine oil, yields nonanal and O=CH(CH2)3COOH on ozonolysis. What is the structure of this acid? 14. Whene levulinic acid (CH3CO-CH2CH2COOH) was hydrogenated at high pressure over a nickel catalyst at 220 ºC, a single product, compound A (C5H8O2), was isolated in 94% yield. Compound A lacks hydroxyl absorption in its infrared spectrum and does not immediately liberate carbon dioxide on being shaken with sodium bicarbonate. What is a reasonable structure for compound A? 15. On standing in dilute aqueous acid, compound B is smoothly converted to mevalonolactone. O CH3 O O H3O CH3 O CH3 OH COOH mevalonolactone compound A Suggest a reasonable mechanism for this reaction. What other organic product is also formed? 3