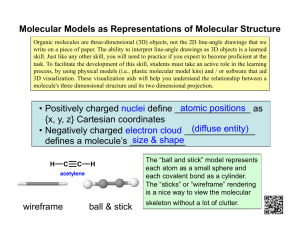
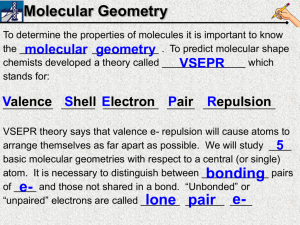
Document
advertisement

CHM 134 General Chemistry I Exam 3 Fall 2005 Name KEY Section I: Each question in this section is worth 2 points. 1. What is the mass of one mole of sodium hydroxide? 40.00 g 2. A silicon atom has 4 valence electrons. 3. Which combination of atoms is most likely to produce a compound with ionic bonds? a) Al and F 4. How many grams are in 0.0751 mol Br2? e) 12.0 g 5. In molecules, as bond order increases, e) bond length decreases and bond energy increases. 6. Predict which of the following compounds will have the most polar covalent bond(s). a) HF 7. What is the molecular geometry around a central atom that is sp3 hybridized and has one lone electron-pair? d) trigonal-pyramidal 8. What is the mass percent of chlorine in FeCl2? d) 55.9% 9. Which of the underlined atoms (C1, C2, N, and O) are sp2 hybridized? .. a) C1 and C2 OH H H .. .O . C1 H H C C NH2 H C C2 C C C H H C H 3 10. What is the molecular geometry around a central atom that is sp d hybridized and has three sigma bonds and two lone electron-pairs? e) T-shaped 11. How many moles are in 35.7 g of copper (II) sulfate? b) 0.224 mol 12. Which of the following are possible Lewis structures for C2H6O? H H H C H H .. C O .. H H .. H C O .. C H H H H H H (2) (1) .. H C C O .. H H H (3) d) 2 and 3 13. According to VSEPR theory, the electron-pair geometry of ICl3 is trigonal bipyramidal and its molecular geometry is T-shaped. 14. What is the formal charge on each atom in a hypobromite ion, OBr-? d) O = -1, Br = 0 15. Which one of the following molecules has a dipole moment (is polar)? c) NCl3 16. If 1.00 g of an unknown molecular compound contains 4.55 × 1021 molecules, what is its molar mass? e) 132 g/mol 17. Which of the following are resonance structures for the formate ion, HCO2-? .. O .. . C O .. . H (1) c) 3 and 4 - .. O .. C O.. H (2) - .. O .. . C O .. . H (3) - .. O .. C O.. H (4) - 18. How many sigma (σ) bonds and pi (π) bonds are in the following molecule? H H C C H C C H e) seven σ and three π 19. One product of the combustion of ethylene, C2H4, is carbon dioxide. What change in hybridization of the carbon occurs in this reaction? H H C C H O C O H ethylene carbon dioxide e) sp2 to sp 20. How many oxygen atoms are in 1.50 grams of SO3? 3.38 x 1022 O atoms Section II: Show your work to solve each question. 21. (4 points) The density of carbon tetrachloride is 1.59 g/mL. How many chlorine atoms are present in 55.0 mL of carbon tetrachloride? 1.37 x 1024 Cl atoms 22. (4 points) A molecule is found to contain 47.35% C, 10.60% H, and 42.05% O. What is the empirical formula for this molecule? The formula is C3H8O2 23. (14 points) Draw a molecular orbital diagram for the diatomic molecule, O2. Label all of the energy levels (both occupied and unoccupied). Show the population of each level with arrows. Use the diagram to answer the following questions. Make sure you explain your answers. What is the bond order of O2? 2 Is O2 diamagnetic or paramagnetic? Paramagnetic, since there are unpaired electrons. Which species has the shortest bond length (strongest bond)? a) O22+ Since you are removing electrons from an antibonding orbital, the bond strengthens. 24. (4 points) Draw the five possible resonance structures of the phosphate ion, PO43-. In each case the phosphorus atom is the central atom of the molecule. 3- 3- O O 3- O P O O O P O 3- O O O P 3- O O O O O O P O O P O 25. (4 points) Draw an appropriate Lewis dot structure for each molecule: (CH3)(CH2)(CO)(OH) Cl2CO O H C Cl Cl H H O C C C H H O H O Section IV: Complete the table below. Each blank is worth 2 points. (30 points total) Formula Lewis Structure Geometry Hybridization on central atom see-saw sp3d trigonal pyramidal sp3 trigonal bipyramidal sp3d tetrahedral sp3 linear sp F F SF4 F S F 1+ H3O1+ H O H H F F PF5 F P F F 1- O ClO41- O Cl O O CS2 S C S