Test format

Chemistry II – AP
Chapter 8 & 9
TEST – Thursday, December 01, 2011
Test format
25 multiple choice (2 points each; 50 points)…See Below
Born-Haber Cycle problem (5 points) o Write out each step, and include the
H, brief description of the step o Solve either for lattice energy, or
H f
for an ionic compound o Table of data will be provided to find necessary energies/enthalpies
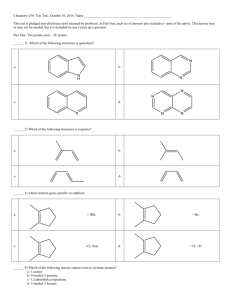
VSEPR table (4, 5-7 points each) o May be one structure that has more than one central atom
Electron-dot structure
- note if/how many
resonance structures
Hybridization Molecular Geometry
- name
- drawing
- bond angles
Polarity
- for each bond
- for the molecule
Additional question(s)
- formal charge
- sigma/pi
- delocalized bonding
- bond angle distortions
- exceptions to octet rule
Topics of questions/problems you can expect:
Chapter 8
Lattice energies, factors o Charge of ions o Distance
Electron configuration of ions
Bond spectrum o Nonpolar covalent (<0.4) o Polar covalent o Ionic (>2.0)
Lewis structures o Octet rule
Exceptions
Less than an octet
More than an octet
Odd number of electrons o Resonance
Formal charge
Bond length/strength (single, double, triple)
H rxn
from bond enthalpies
Chapter 9
VSEPR, Molecular geometry o Bonding/Nonbonding pairs o Geometry; electron-domain geometry & molecular geometry o Bond angles, any distortion to geometrically predicted bond angles? o Hybridization
Polarity of molecules
Sigma/pi bonds
Delocalized bonding/electrons