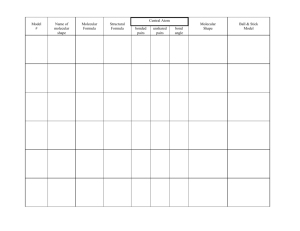
Shapes of molecules - chemistryatdulwich
advertisement
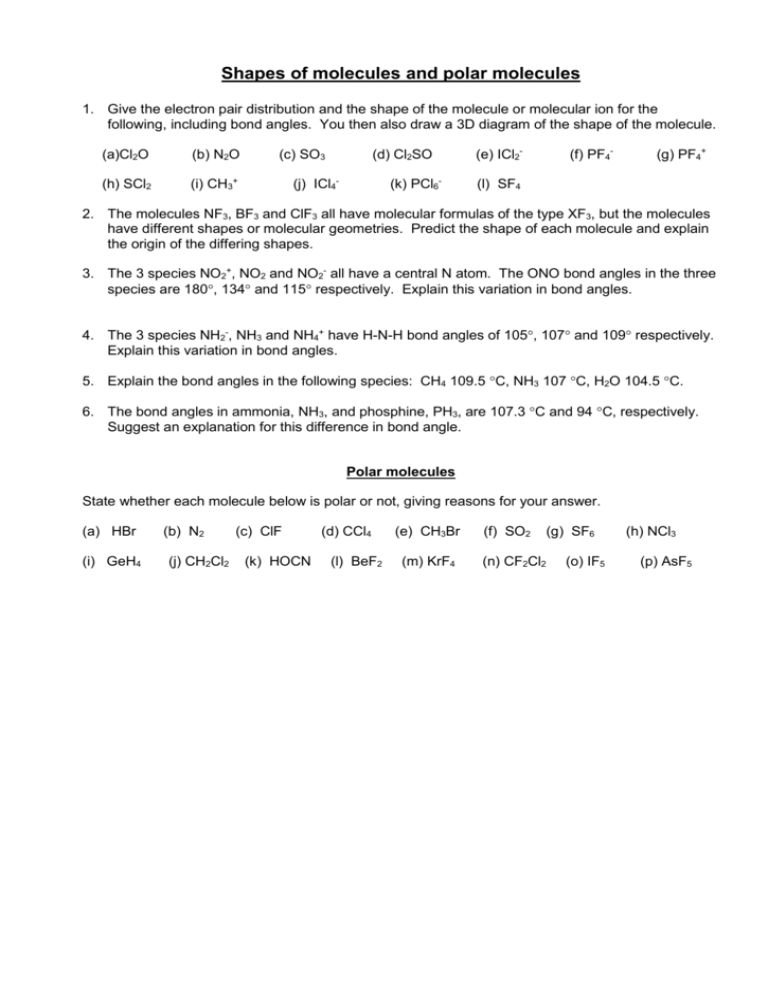
Shapes of molecules and polar molecules 1. Give the electron pair distribution and the shape of the molecule or molecular ion for the following, including bond angles. You then also draw a 3D diagram of the shape of the molecule. (a)Cl2O (b) N2O (h) SCl2 (i) CH3+ (c) SO3 (d) Cl2SO (j) ICl4- (k) PCl6- (e) ICl2- (f) PF4- (g) PF4+ (l) SF4 2. The molecules NF3, BF3 and ClF3 all have molecular formulas of the type XF3, but the molecules have different shapes or molecular geometries. Predict the shape of each molecule and explain the origin of the differing shapes. 3. The 3 species NO2+, NO2 and NO2- all have a central N atom. The ONO bond angles in the three species are 180, 134 and 115 respectively. Explain this variation in bond angles. 4. The 3 species NH2-, NH3 and NH4+ have H-N-H bond angles of 105, 107 and 109 respectively. Explain this variation in bond angles. 5. Explain the bond angles in the following species: CH4 109.5 C, NH3 107 C, H2O 104.5 C. 6. The bond angles in ammonia, NH3, and phosphine, PH3, are 107.3 C and 94 C, respectively. Suggest an explanation for this difference in bond angle. Polar molecules State whether each molecule below is polar or not, giving reasons for your answer. (a) HBr (i) GeH4 (b) N2 (j) CH2Cl2 (c) ClF (k) HOCN (d) CCl4 (l) BeF2 (e) CH3Br (m) KrF4 (f) SO2 (n) CF2Cl2 (g) SF6 (o) IF5 (h) NCl3 (p) AsF5