Lewis Structures, Molecular Shape & VSEPR Worksheet
advertisement

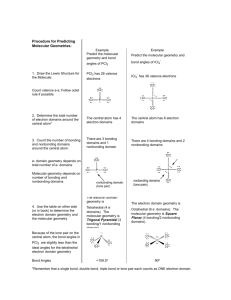
Lewis Structures, Molecular Shape and VSEPR The Valence Shell Electron Pair Repulsion theory (VSEPR) allows one to predict the shape of a molecule from the Lewis electron dot structure. The theory is based on the assumption that the electron pairs surrounding the central atom repel each other and distance themselves in the surrounding space with an arrangement which reduces the amount of repulsions. Such spatial arrangements can be predicted from a knowledge of the number of electron groups surrounding a central atom. The table at the right summarizes the results of the theory. When determining the number of electron groups, a single bond, a multiple bond and a non-bonding pair are all counted as a single electron group. The electron pair geometry about the central atom is directly effected by the number of electron groups. The molecular shape is determined by the arrangement of bonded atoms about the central atom of the molecule. If all electron groups are involved in bonding, then the molecular shape and the electron pair geometry are identical. When an electron group is a non-bonding pair, the molecular shape can be determined by visually removing an atom from one of the corners of the electron pair geometry. Student mastery of VSEPR is dependent upon: • an ability to draw Lewis structures • an understanding of the table at the right and a willingness to use it • an ability to spatially visualize geometric arrangements • a willingness to use your noodle • patience and hard work From Zumdahl, Chemistry, 6th edition For the following listed compounds, draw the Lewis Dot structure in order to determine the number of electron groups, the number of bonding and non-bonding groups and the resulting electron pair geometry and molecular geometry. Finally, approximate the value of the bond angles. Comp'd 1. CH4 2. BF3 3. N2O (NNO) 4. Br2 5. SF4 6. HCl Lewis Dot Structure # of eGroups # of Bonding # of NonPairs Bonding Pairs e- Pair Geometry Molecular Geometry ~ Bond Angles Polarity Comp'd 7. PH3 8. H 3O + 9. SO3 10. CO2 11. XeCl2 12. SO2 Lewis Dot Structure # of eGroups # of Bonding # of NonPairs Bonding Pairs e- Pair Geometry Molecular Geometry ~ Bond Angle Polarity Comp'd 13. SF6 14. PCl5 15. SeO2 16. ClO2- 17. ClF2- 18. O3 Lewis Dot Structure # of eGroups # of Bonding # of NonPairs Bonding Pairs e- Pair Geometry Molecular Geometry ~ Bond Angle Polarity