Answers
advertisement
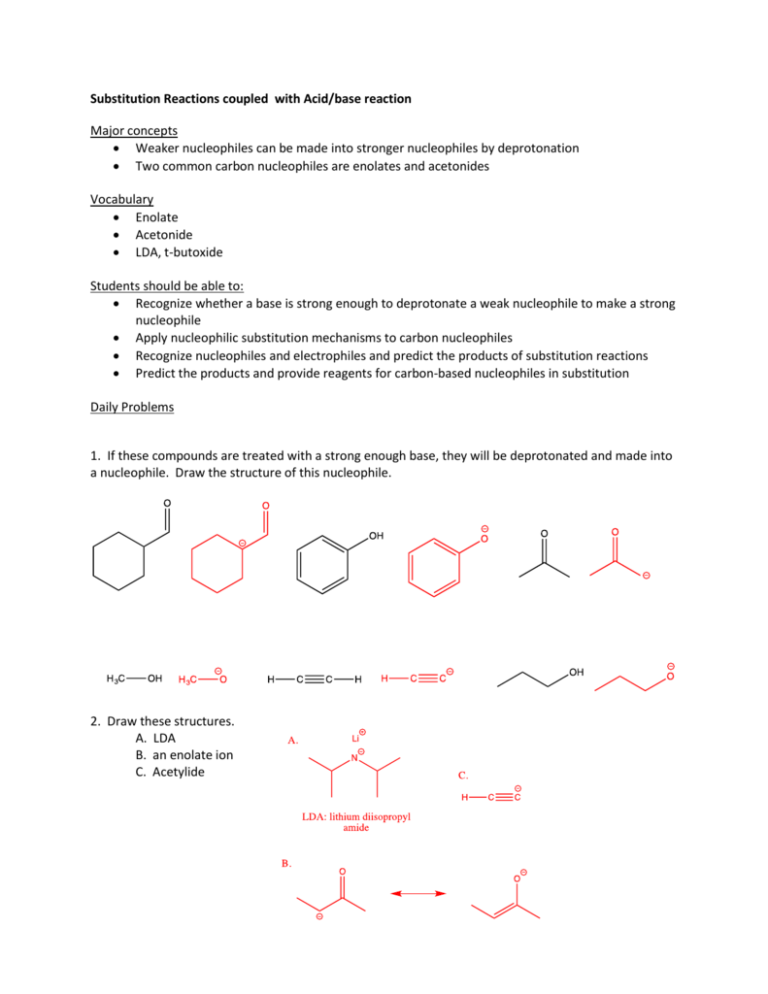
Substitution Reactions coupled with Acid/base reaction Major concepts Weaker nucleophiles can be made into stronger nucleophiles by deprotonation Two common carbon nucleophiles are enolates and acetonides Vocabulary Enolate Acetonide LDA, t-butoxide Students should be able to: Recognize whether a base is strong enough to deprotonate a weak nucleophile to make a strong nucleophile Apply nucleophilic substitution mechanisms to carbon nucleophiles Recognize nucleophiles and electrophiles and predict the products of substitution reactions Predict the products and provide reagents for carbon-based nucleophiles in substitution Daily Problems 1. If these compounds are treated with a strong enough base, they will be deprotonated and made into a nucleophile. Draw the structure of this nucleophile. 2. Draw these structures. A. LDA B. an enolate ion C. Acetylide 3. Provide an arrow mechanism with all intermediate structures for the following substitution reaction. 4. In the table, put a check next to each base that is capable of completely deprotonating the compound to make it a good nucleophile. pKa of conj acid pKa 18 pKa 35 pKa 36 pKa Potassium t-butoxide Sodium hydride OH 10 O 20 H H3C O C CH 25 X X O <20 OH X 16 5. Predict the major product(s) of the following substitution reactions. LDA 6. Provide the necessary reagents. 1. NaH 2. MeI 1. KOH 2. H3CH2CBr 1. LDA 2. MeI Cumulative Problems 7. Label each reaction as addition, elimination, or substitution if it is not already given. Predict the major product of each of these reactions. elimination addition 8. Explain why these reactions will not take place as written. The electrophilic carbon is tertiary or too sterically hindered to react in a substitution. The product from the elimination of the other beta H would lead to conjugation. Addition of water would make the addition reaction (equilibrium to the left) more favorable. Substitution reactions causes inversion of stereochemistry. Unfavorable reaction based on the poor leaving group. 9. Propose a substitution reaction that would lead to the formation of SAM. Draw the necessary arrows. (Hint: Indicate the new bond in the product. Which two atoms are now connected? Which was the electrophile? Which was the substrate?) Why would SAM be a good methylating reagent? (Why is it willing to donate a methyl group to another molecule?) SAM is a good methylating reagent because the positive sulfur want another lone pair (typically bonded sulfurs are like typically bonded O in that they want two lone pairs and two bonds); therefore, it is a good leaving group.