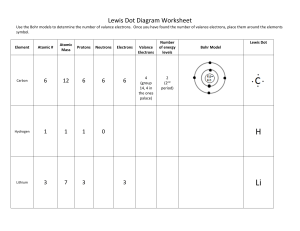
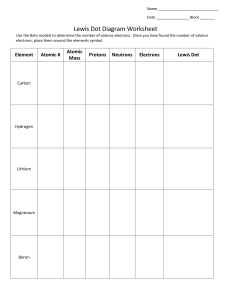
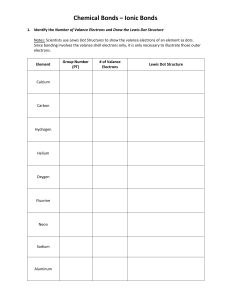
SNC 1D Chemical Bonds – Ionic Bonds 1. Identify the Number of Valance Electrons and Draw the Lewis Dot Structure Notes: Scientists use Lewis Dot Structures to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons. Element #of valence electrons Tendency to lose or gain Calcium 2 lose (2) Chlorine 7 gain (1) Lewis Dot Structure Magnesium Mg Sulfur S Lithium Li Oxygen O Aluminum Al Iodine I 2. Draw the formation of the ionic compounds using Lewis Dot Diagrams for the following pairs of elements. The first example is done for you. 1. Calcium and Chlorine → 2. Magnesium and Sulfur 3. Lithium and Oxygen 4. Aluminum and Iodine