ICP-OES and ICP-MS Detection Limit Guidance BR023
advertisement

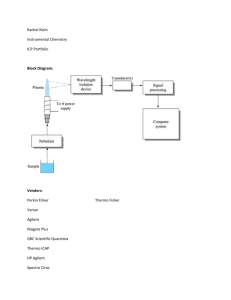
ICP-OES and ICP-MS Detection Limit Guidance Group 1 IA 1 4 Li 10 5 0.1 0.05 Period 4 Mg 5 25 1 0.5 2.5 0.1 0.05 20 K Ca 100 50 1 0.5 37 5 38 Rb Sr 50 25 0.1 0.05 5 2.5 0.1 0.05 55 6 56 Cs 40000 20000 0.1 0.05 ICP-OES (µg/L) ICP-OES (µg/g) ICP-MS (µg/L) ICP-MS (µg/g) 4 IVB 22 5 VB 23 6 VIB 24 5 2.5 0.1 0.05 5 2.5 0.1 0.05 5 2.5 0.1 0.05 5 2.5 0.1 0.05 Sc 39 Li 10 5 0.1 0.05 Ti 40 Y Zr 5 2.5 0.1 0.05 5 2.5 0.1 0.05 72 Hf 50 25 0.1 0.05 Ra 3 Symbol 3 IIIB 21 88 Atomic Number V 41 Nb 50 25 0.1 0.05 73 Ta 50 25 0.1 0.05 Cr 42 Mo 10 5 0.1 0.05 74 W 50 25 0.1 0.05 7 VIIB 25 Mn 5 2.5 0.1 0.05 43 Tc 75 Re 10 5 0.1 0.05 8 26 Fe 44 5 2.5 1 0.5 Ru 50 25 0.1 0.05 76 Os 5 2.5 0.1 0.05 9 VIII 27 Co 5 2.5 0.1 0.05 45 Rh 100 50 0.1 0.05 77 Ir 50 25 0.1 0.05 10 28 Ni 5 2.5 0.1 0.05 46 Pd 50 25 0.1 0.05 78 Pt 50 25 0.1 0.05 11 IB 29 Cu 5 2.5 0.1 0.05 47 Ag 5 2.5 0.1 0.05 79 Au 50 25 0.1 0.05 12 IIB 30 Zn 5 2.5 0.1 0.05 48 Cd 5 2.5 0.1 0.05 80 Hg 50 25 0.1 0.05 106 107 108 109 110 111 112 57 58 59 60 61 62 63 64 65 La 10 5 0.1 0.05 89 Ac Db Ce 50 25 0.1 0.05 90 Th 50 25 0.1 0.05 Sg Pr 50 25 0.1 0.05 91 Pa Bh Hs Mt Nd Pm Sm 50 50 25 0.1 0.05 92 U 500 250 0.1 0.05 93 Np 25 0.1 0.05 94 Ds Eu 10 5 0.1 0.05 95 Rg Gd 50 25 0.1 0.05 96 B 10 13 105 Rf 13 IIIA 5 0.1 0.05 104 www.eag.com/mc Copyright ©2014 Evans Analytical Group • 05/14 BR023 5 prep volume These detection limits are theoretical best case scenarios assuming there are no spectral interferences affecting the best isotope or wavelength for a given element. For any given determination, the actual method detection limit can be an order of magnitude higher or more. Use this as a guide, not absolute information. 5 2.5 0.1 0.05 Fr 50 ml [sol conc (µg/L) * prep vol (L)] / sample mass (g) = sample concentration in ppm wt (µg/g) Ba 87 7 5 2.5 1 0.5 sample mass To convert mg/L to ppm wt, use the following calculation: 12 Na 50 19 0.1 g 2.5 0.1 0.05 11 3 Be 5 Lanthanides 3 2 IIA Actinides 1 2 2 H Al 20 10 0.1 0.05 31 Ga 50 25 0.1 0.05 49 In 50 25 0.1 0.05 81 Tl 50 25 0.1 0.05 Cn Tb 50 25 0.1 0.05 97 Pu Am Cm Bk 6 14 IVA C 14 Si 20 32 10 1 0.5 Ge 50 25 0.1 0.05 50 Sn 50 25 0.1 0.05 82 Pb 50 25 0.1 0.05 114 7 15 VA N 15 33 P 100 50 1 0.5 As 50 25 0.1 0.05 51 Sb 50 25 0.1 0.05 83 Bi 100 50 0.1 0.05 Fl 66 Dy 10 5 0.1 0.05 98 Cf 67 Ho 10 5 0.1 0.05 99 Es 8 16 VIA O 16 S 34 Se 50 25 0.1 0.05 52 Te 50 25 0.1 0.05 84 Po 9 17 VIIA F 17 Cl n/a 35 n/a 10 5 Br 53 85 n/a n/a 1 0.5 I n/a n/a 1 0.5 At 18 VIIIA He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn 116 Lv 68 Er 10 5 0.1 0.05 100 69 Tm 10 5 0.1 0.05 101 Fm Md 70 Yb 10 5 0.1 0.05 102 No 71 Lu 10 5 0.1 0.05 103 Lr Inductively Coupled Plasma Spectroscopy The Inductively Coupled Plasma Spectroscopy techniques typically analyze materials in liquid form. In plasma emission spectroscopy (OES), a sample solution is introduced into the core of an inductively coupled argon plasma (ICP), which generates a temperature on the order of 10,000°C. At this temperature all elements become thermally excited and emit light at their characteristic wavelengths. This light is collected by the spectrometer and passes through a diffraction grating that serves to resolve the light into a spectrum of its constituent wavelengths. Within the spectrometer, this diffracted light is then collected by wavelength and amplified to yield an intensity measurement that can be converted to an elemental concentration by comparison with calibration standards. In plasma mass spectroscopy (MS), the inductively coupled argon plasma (ICP) is once again used as an excitation source for the elements of interest. However in contrast to OES, the plasma in ICP-MS is used to generate ions that are then introduced to the mass analyzer. These ions are then separated and collected according to their mass to charge ratios. The constituents of an unknown sample can then be identified and measured. ICP-MS offers extremely high sensitivity to a wide range of elements. Strengths Limitations Up to 70 elements can be determined simultaneously in a single sample analysis. The emission spectra are complex and inter-element interferences are possible if the wavelength of the element of interest is very close to that of another element. The useful working range is over several orders of magnitude. In mass spectrometry, determination and quantification of certain elements can be affected by interference from polyatomic species, matrix elements and atmospheric elements. Instrumentation is suitable to automation, thus enhancing accuracy, precision and throughput. The sample to be analyzed must be digested prior to analysis in order to determine the element(s) of interest. ICP-MS ICP-OES I+ liquid liquid plasma www.eag.com/mc Copyright ©2014 Evans Analytical Group • 05/14 BR023 hν I+ plasma hν