ICP-MS
advertisement

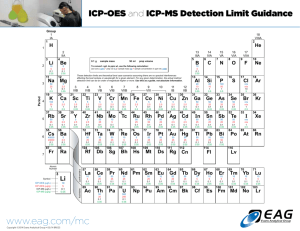
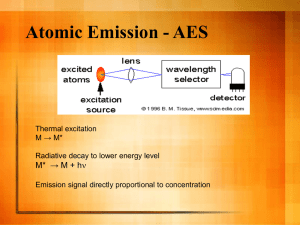
Class Data: Cross Country Field – Core 3 Profile δ 13C (‰ vs.VPDB) Gr. 3-5A Gr. 3-15 Gr. 3-25 Gr. 3-45 Gr. 3-55 Gr. 3-61 Gr. 3-70 Gr. 3-85 Gr. 3-92 Gr. 3-100 Gr. 3-110 Gr. 3-120 Gr. 3-135 Gr. 3-145 Gr. 3-159 Depth δ13C (cm) (‰) 5 -24.70 15 -24.14 25 -24.05 45 -23.71 55 -23.60 61 -23.47 70 -23.43 85 -24.28 92 -23.98 100 -24.30 110 -24.31 120 -24.79 135 -24.54 145 -24.53 159 -24.85 mean -24.18 st dev 0.47 Carbon Nitrogen (%) (%) 1.62% 0.17% 1.02% 0.12% 0.71% 0.09% 0.38% 0.05% 0.32% 0.05% 0.37% 0.05% 0.39% 0.06% 0.34% 0.05% 0.22% 0.03% 0.23% 0.04% 0.14% 0.03% 0.10% 0.03% 0.11% 0.03% 0.11% 0.03% 0.11% 0.03% δ13C Depth: (‰) (cm) -24.70 -5 -24.14 -15 -24.05 -25 -23.71 -45 -23.60 -55 -23.47 -61 -23.43 -70 -24.28 -85 -23.98 -92 -24.30 -100 -24.31 -110 -24.79 -120 -24.54 -135 -24.53 -145 -24.85 -159 -25.0 0 -24.5 -24.0 -23.5 -23.0 -20 -40 -60 Depth (cm) ID -80 -100 -120 -140 -160 -180 ? Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) ICP-OES has been widely used since the 1970's. Instrument Description and Theory ICP technology was built upon the same principles used in atomic emission spectrometry. Samples are decomposed to neutral elements in a high temperature argon plasma and analyzed based on their mass to charge ratios. An ICP-MS can be thought of as four main processes, including sample introduction and aerosol generation, ionization by an argon plasma source, mass discrimination, and the detection system. The schematic below illustrates this sequence of processes. Plasma is the phase of matter with its electrons stripped. In argon plasma, argon ions and electrons act as the conducting species. Three power sources are dc-electric, radio and microwave frequency generators. The most advantageous is the radio or inductively coupled plasma (ICP) because of sensitivity and minimal interference. DC plasma source (DCP) are also advantageous and is also simple and less expensive. Inductive Coupled Plasma consist of three concentric quartz tubes in which streams of argon flow. Ionization of the argon is initiated by a spark from a Tesla coil. The geometries of CP source, in radial geometry or axial geometry Inductively coupled plasma mass spectroscopy (ICP-MS) was developed in the late 1980's to combine the easy sample introduction and quick analysis of ICP technology with the accurate and low detection limits of a mass spectrometer. Figure 3. Schematic of quadrupole mass filter. (Figure reproduced with permission from PerkinElmer, Inc.) analysis Figure 2. The interface region of an ICP-MS. (Figure reproduced with permission from PerkinElmer, Inc.) sample introduction Figure 1. The ICP Torch showing the fate of the sample. (Figure reproduced with permission from PerkinElmer, Inc.) The resulting instrument is capable of trace multielement analysis, often at the part per trillion level. ICP-MS has been used widely over the years, finding applications in a number of different fields including drinking water, wastewater, natural water systems/hydrogeology, geology and soil science, mining/metallurgy, food sciences, and medicine. An ICP-MS combines a high-temperature ICP (Inductively Coupled Plasma) source with a mass spectrometer. Figure 1. The ICP Torch showing the fate of the sample. (Figure reproduced with permission from PerkinElmer, Inc.) The most important things to remember about the argon ICP plasma are: •The argon discharge, with a temperature of around 6000-10000°K, is an excellent ion source. •The ions formed by the ICP discharge are typically positive ions, M+ or M+², therefore, elements that prefer to form negative ions, such as Cl, I, F, etc., are very difficult to determine via ICP-MS. •The detection capabilities of the technique can vary with the sample introduction technique used, as different techniques will allow differing amounts of sample to reach the ICP plasma. •Detection capabilities will vary with the sample matrix, which may affect the degree of ionization that will occur in the plasma or allow the formation of species that may interfere with the analyte determination. What can be analyzed? •Water samples are typically analyzed without sample preparation if they have been filtered and acidified during collection. •Sediment, soil and rock samples for total elemental analysis are digested using a 4-acid digestion procedure in order to dissolve most silicate minerals. This digestion is carried out in open vessels on a hot-plate, so if volatile elements are of interest, another digestion procedure such as microwave digestion should be used. •Geological samples for rare-earth-element (REE) analysis are typically prepared using a sodium peroxide sinter method. In this method the ground sample is mixed with sodium peroxide in a carbon crucible and placed in a muffle furnace. The resulting sinter is leached with water and acidified with nitric acid before analysis. •Biological and organic samples are generally digested using a closed-vessel microwave digestion procedure that is appropriate to the matrix of the sample. This is also the best method for digesting organic samples, including crude oils. •Speciation Analysis is performed on a variety of sample types using High Performance Liquid Chromatography (HPLC) to separate different chemical forms of an element followed by ICP-MS detection. Sample collection, storage, and pretreatment steps are highly specialized. Photographs of argon plasma in operation & ICP torch body. Cyclonic nebulizer in front of torch body. Schematic of ICP-MS main processes. torch detector nebulizer http://www.seaes.manchester.ac.uk/research/facilities/agu/equipment/ICP_MS/moreinfo/ Table 1. Recommended wavelengths and estimated instrumental detection limits • The wavelengths listed are recommended because of their sensitivity and overall acceptance. • Other wavelengths may be substituted if they can provide the needed sensitivity and are treated with the same corrective techniques for spectral interference. Element Aluminum Antimony Arsenic Barium Beryllium Boron Cadmium Calcium Chromium Cobalt Copper Iron Lead Magnesium Manganese Molybdenum Nickel Potassium Selenium Silicon Silver Sodium Thallium Vanadium Zinc Estimated Wavelength Detection (nm) Limit (µg/L) 308.215 45 206.833 32 193.696 53 455.403 2 313.042 0.3 249.773 5 226.502 4 317.933 10 267.716 7 228.616 7 324.754 6 259.940 7 220.353 42 279.079 30 257.610 2 202.030 8 231.604 15 766.491 variable 196.026 75 288.158 58 328.068 7 588.995 29 190.864 40 292.402 8 213.856 2 Inductively Coupled Plasma - (ICP) Strengths • Up to 70 elements can be determined simultaneously in a single analysis. • The useful working range is over several orders of magnitude. • Instrumentation is suitable to automation. Limitations • The emission spectra are complex and inter-element interferences are possible if the wavelength of the element of interest is very close to that of another element. • During Mass Spectrometry, the common matrix elements and other molecular species can interfere with the measurement of some elements. Doubly charged or molecular ionic species can create difficulties in quantifications. • The sample to be analyzed must be digested prior to analysis in order to dissolve the element(s) of interest. Graphite Furnace Atomic Absorption Spec. (GFAA) Graphite Furnace 2400 oC