Document
advertisement

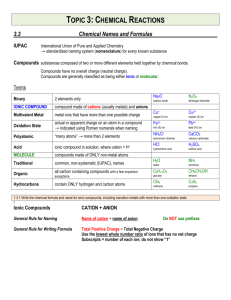
U7D2 Writing and Naming Ionic Formulas U7D2: Writing and Naming Ionic Formula HW: WS: Formula Writing and Naming Practice Do Now: 1.HW out for check 2. Is [Cd]-2 the correct ion dot diagram for a Cadmium ion? Explain. Today: • Review • Naming Ionic Compounds • Practice!!! • TTL The three steps in forming an ionic bond are: Metal loses e- to nonmetal becoming a cation. 1. ____________________________________________ Nonmetal gains e- from metal to become an anion. 2. ____________________________________________ Cation and anion attract to form an ionic compound. 3. ____________________________________________ Period 1 Let’s practice More???? • So.. Fist to Five Fist (not understanding need help) Five (feel I can teach it) • Show me where you stand Ionic Formula Writing • The number of each type of ion that will form the new compound will depend on the size or magnitude of the charges (oxidation states). • The charges must add up to zero. • Ex. sodium reacts with chlorine, • sodium forms a Na+1 ion & chlorine forms a Cl-1 ion. • Only one of each ion is necessary -> • the charges add up to zero: (+1) + (-1) =0 the formula of the new compound, sodium chloride, is simply NaCl. but what if the charges aren’t equal and opposite? • If sodium reacts with oxygen, • sodium still forms a Na+1 ion but oxygen forms a O-2 ion. • The new compound, sodium oxide, will require 2 Na+1 for every O-2: 2(+1) + (-2) =0 . • The formula for sodium oxide is Na2O. Remember: • the charges are written as superscripts (Na+1) and the counters in formulas are written as subscripts (Na2O). • Positive ions are written first and negative ions are written second in a formula. • Lazy chemists do not bother to write 1 as counter in a formula. The element's symbol stands for the 1. • Ionic formulas are always written in lowest terms. => an empirical formula. • NaCl and Na2O are binary (2 element) ionic cds. VI. DETERMINING CHARGES: • The oxidation state or number is the charge or apparent charge an atom has in a compound. • To determine the charge, simply look at the Periodic Table for the oxidation states of the element. • For nonmetals it is the FIRST (top) oxidation state ONLY. • For many metals, there is only one possibility for the charge. • Transition metals and those metals close to the “crack”, there are more than one charge. Look up the charges on the following: • Remember to write the charges as superscript Sr+2 -2 O Ag Cl +1 +2 Fe +3 Fe +1 Cu -1 +2 Cu -3 N P +3 Cs +1 -1 Br Draw a Lewis Dot structure for (p. 3 margin) FeO and Fe2O3 WRITING BINARY FORMULAS • The ions of the elements combine in such a way that the charges have to add up to zero. • Metal or positive ion (cation) is written FIRST • Nonmetal or negative ion(anion)is written SECOND. • The number one,1, is not written in the formula ex. NaCl. • Only the number in the charge for each ion is criss- crossed down and turned into subscripts. • Always check that the formula is in LOWEST terms. • K+1 O-2 K O K2O1 or K2O PbO2 • Pb+4 O-2 -> Pb O -> Pb2O4 simplifies to ______ So… Let’s look at the different combinations we can have… Ions to be Bonded How to determine formula X+1 & Y-1 +1 and -1 add up to zero already XY X+2 & Y-2 +2 and -2 add up to zero already XY +3 and -3 add up to zero already XY It takes two -1 charges to cancel out the +2 charge It takes three -1 charges to cancel out the +3 charge XY2 +3 X &Y -3 X+2 & Y-1 X+3 & Y-1 Formula XY3 Ions to be Bonded X+1 & Y-2 How to determine formula It takes two +1 charges to cancel out the -2 charge +1 -3 It takes three +1 charges to cancel out X &Y the -3 charge +2 -3 Common denominator: 2x3=6 it takes X &Y three +2 to make +6 and two -3 to make -6 X+3 & Y-2 Common denominator: 2x3=6 it takes two +3 to make +6 and three -2 to make -6 +4 -2 It takes two -2 charges to cancel out the X &Y +4 charge Formula X2Y X3Y X3Y2 X2Y3 XY2 VII. NAMING • The name of the positive ion is followed by the name of the negative ion. • Group 1 & 2 Metals, Metals with ONE charge LISTED: name of the metal ONLY • For nonmetals, the ending of the nonmetal’s name with the ending changed to IDE. Let’s try! • Name ionic compounds: • NaF: sodium fluoride • AlN: Aluminum nitride • AgBr: Silver bromide • Na3P: sodium phosphide • Li2O: Lithium oxide • PbCl2 : Lead (II) chloride Practice ex. 1.) 2.) 3.) 4.) 5.) Cation Mg+2 +1 Li Al+3 Sr+2 K+1 Mg+2 Anion Formula Name Br-1 MgBr2 Magnesium Bromide -1 LiF Lithium fluoride F AlCl3 Aluminum chloride Cl-1 Strontium sulfide SrS S-2 Potassium nitride K3N N-3 Magnesium phosphide Mg3P2 P-3 How about backwards? Name -> formula • Determine the elements involved, their charges & then write the formulas. Look up charges on the PT for the ions. Name Cation Anion Formula Na+1 NaBr Br-1 1.) Sodium Bromide 2.) Calcium Oxide Ca+2 O-2 CaO 3.) Zinc Chloride Zn+2 Cl-1 F-1 ZnCl2 I-1 KI 4.) Beryllium Fluoride 5.) Potassium Iodide Be+2 K+1 BeF2 VIII. DETERMINING CHARGES AND NAMING of TRANSITION ELEMENTS • Transition elements (metals) and some other metals located near the “crack”, can have more than one charge. • Either you will be told which one to use or the charge can be determined from the formula. • To distinguish between the different oxidation states or charges, a roman numeral equal to the charge on the ion is used to name the ion. • Ex, tin comes in two charges, +2 and +4. • Sn+2 is called tin (II) and Sn+4 is called tin (IV). • The formula of tin (II) oxide is SnO and • the formula of tin (IV) oxide is SnO2. • The charge on the tin makes a difference! • If Fe2O3 is iron (III) oxide, what would be the FeO formula for iron (II) oxide? _____ • (Draw the Ion dot diagram for Iron (II) oxide) ROMAN NUMERALS: III +4 ____, VI IV +5 ____, +1 ___, +3 ____, I +2 ____, II V +6 _____ Write the formula and name of these compounds with transition metals: Cation Anion Formula Name +2 -1 ex. Pb Cl PbCl2 lead (II) chloride +1 -2 1. Cu O Cu2O Copper (I) oxide 2. Ni+3 S-2 Ni2O3 Nickel (III) sulfide 3. Fe+2 I-1 FeI2 Iron (II) iodide 4. Au+1 N-3 Au3N Gold (I) nitride 5. Pb+4 O-2 PbO2 Lead (IV) oxide Going Backwards! Roman numerals tell you what charge to use for the metal (first element): gold (III) chloride tells you to use Au+3 and NOT Au+1 Name ex. Iron (II) iodide Cation Anion Formula Fe+2 I-1 BaI2 1. Copper (I) fluoride Cu+1 F-1 CuF 2. Copper (II) chloride Cu+2 Cl-1 CuCl2 3. Gold (III) oxide Au+3 O-2 Au2O3 4. Tin (IV) oxide Sn+4 O-2 SnO2 5. Lead (II) phosphide Pb+2 P-3 Pb3P2 Naming the Ionic Compounds from JUST the Formula • Determine if it has a metal with more than 1 charge or not • If yes, then look up the nonmetal’s charge and write the formula • • • • • • • • • • with each of the charges. Ex . FeO Fe+2 O-2 FeO Fe+3 O-2 Fe2O3 a) Since the formula with Fe+2 matches, FeO, then Fe+2 was used. Use the Roman numeral equal to this charge in the middle of the compound’s name. Iron (II) b) Change the name of the non-metal to the ending “ide”. Oxide Iron (II) Oxide If no, then just use the metal’s name and change the name of the nonmetal to the ending “ide”. Ex. AgCl Ag+1 +1 is the only charge Silver Chloride • Remember any element in Groups 1 & 2 have ONLY one Charge!! No Roman Numeral ever!!! Formula Name Formula Name AgCl Fe2O3 Iron (III) oxide PbO2 SnO Silver chloride AuI 3 Lead (IV) oxide CrN Tin (II) oxide Gold(III) iodide Chromium(III) Nitride Na3P Sodium phosphide CuO Copper(II) oxide ZnBr2 Calcium nitride Zinc bromide Ca3N2 IX. POLYATOMIC IONS (PAI) • A. Reference Table E • Groups of atoms bond together by sharing electrons to form ions. • one of the atoms in the group brings along a charge and group of atoms is not neutral • “many atom” ion. Complete this chart using reference table E. Polyatomic Ion name ex. 1. Formula Polyatomic Ion Formula Name Ammonium NH4+1 ex. MnO4 - Permanganate -2 6. Nitrate CO carbonate 3 NO 3 7. C H O 2 3 2 ClO2 2. Chlorite 3. Chromate CrO4 -2 8. SO4-2 sulfate 4. Sulfite ClO3- chlorate 5. Phosphate PO4 -3 10. SO3 -2 9. OH - acetate hydroxide • What type of charge do most of the PAI have? __________________ negative -ite -ate • Most of these PAI end in _________ or __________ • Two important exceptions are the positive ion + NH 4 _________ (ammonium) and the negative ion _________ (hydroxide). OH- B. Writing and Naming Compounds with Polyatomic Ions Rules: • The formula must be in lowest terms • the charges must add up to zero. • Parenthesis are used around the polyatomic ion when more than one of these ions is necessary in a formula: (NH4)2O. • To name compounds with polyatomic ions, use the name of the polyatomic ion listed on the reference table. • Ex: (NH4)2O is ammonium oxide; • K2CO3 is potassium carbonate. • ternary ionic compounds: • binary ionic compounds: K2CO3 and NaClO4 K2O and NaCl Try your hand at these! Cation Anion 1. K+1 NO3 -1 2. Al+3 ClO3 -1 Al(ClO3) 3. Sr +2 4. K+1 NO2 -1 5. Mg+2 PO4 Mg+2 ClO-1 SO4 -2 -3 Formula Name Mg(ClO)2 Magnesium hypochlorite KNO3 Potassium nitrate Aluminum chlorate 3 SrSO4 Strontium sulfate KNO2 Potassium nitrite Mg3(PO4)2 Magnesium phosphate Going Backwards! ternary compounds Name Anion Formula ex. Beryllium nitrite Cation 1. Sodium hydroxide Na +1 OH - NaOH 2. Calcium sulfate 3. Be+2 (NO2)-1 Be(NO2)2 Ca +1 SO2 -2 Potassium chromate K +1 CrO -2 4 4. 5. Gold (III) carbonate **Ammonium perchlorate CaSO4 K2CrO4 Au +3 CO3 -2 Au2(CO3)3 NH3 + ClO4 - NH3ClO4 Ok…so how’d we do today 1. • Cation:__Ag+1__ Anion:___ (OH)-1____ • Formula:___________________ • Name:_____________________ • 2. • Cation:____ Fe+3__ Anion:___ (PO4)-3___ • Formula:___________________ • Name:_____________________