Chapter 16 - Chemistry
advertisement

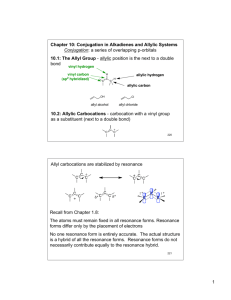
Chapter 16 – Conjugati on, resonance, and dienes Conjugation relies on the partial overlap of p-orbitals on adjacent double or triple bonds. A common conjugated system involves 1,3-dienes, such as 1,3-butadiene. in a conjugated system, overlap of p orbitals allows electrons to be delocalized over a larger portion of the molecule, thus lowering the energy of the molecule and making it more stable However, it is possible for two systems to be in “cross-conjugation” with each other, as in the example below (the two benzene rings are crossconjugated, NOT conjugated!): Conjugation is broken completely by the introduction of saturated (sp3) carbon: The allylic carbocation is another example of a conjugated system – conjugation stabilizes an allylic carbocation (due to two resonance forms) CH2 CH2 - the true structure is a hybrid of the two resonance forms – the π bond is delocalized over all three C atoms and the positive charge is delocalized over the two terminal C’s. Thus, an allylic carbocation is more stable than a normal 1˚ carbocation; its stability is comparable to that of a 2˚ carbocation: CH3 < RCH2 < R2CH ~~ CH2=CH-CH2 < R3C common examples of when resonance structures are drawn for a molecule or reactive intermediate (besides allylic type): 1. Conjugated double bonds - acylic : : H2C CH2 H2C CH2 1 cyclic – e.g. benzene 2. cations having a positive charge next to a lone pair O CH2 H3C O CH2 : : : H3C 3. double bonds having one atom more electronegative than the other H3C H3C C C O O H3C H3C REMEMBER: 1. Resonance structures with more bonds and fewer charges are more stable. 2. Resonance structures in which every atom has an octet are more stable. 3. Resonance structures that place a negative charge on the more electronegative atom are more stable. Which of the following two resonance structures is more stable? : : : HN C C O : : HN O: H3C H3C In any system, X=Y-Z, Z is sp2-hybridized and the nonbonded electron pair occupies a p orbital to make the system conjugated. E.g. : O : : C O: C Conjugated dienes (1,3-dienes) – conformational and stereosiomers, etc. For 1,3-dienes with alkyl groups bonded to each end carbon of the diene, RCH=CH-CH=CHR, 3 stereoisomers are possible: R R R cis,cis isomer or (Z,Z)-1,3 diene R R trans,trans isomer or (E,E)-1,3 diene R cis,trans isomer or (Z,E)-1,3 diene 2 conformational isomers are also possible because two conformations result from rotation around the C-C bond that joins the two double bonds s-cis conformation s-trans conformation e.g. R R R R R R trans,trans isomer s-trans conformation trans,trans isomer s-cis conformation two conformers cis,cis isomer or (Z,Z)-1,3 diene two stereoisomers Four features distinguish conjugated dienes from isolated dienes: 1. The C-C single bond joining the two double bonds is unusually short. Ethylene C=C = 1.34 Å Ethane C-C bond = 1.53 Å 1.34 Å 1.48 Å - the shorter C-C bond in butadiene can be explained by sp2 hybridization of the C atoms or by resonance imparting partial double bond character. 2. Conjugated dienes are more stable than similar isolated dienes – A conjugated diene has smaller heat of hydrogenation than a similar isolated diene H2 (g) Pd-C !H = -61 kccal/mol H2 (g) Pd-C !H = -54 kccal/mol 3. Conjugated dienes absorb at longer wavelengths of uv light (lower energy). 3 4. Some reactions of conjugated dienes are different from reactions of isolated double bonds DO PROBLEMS 16.13 AND 16.14 IN CLASS. Electrophilic Addition: 1,2- versus 1,4- Addition Electrophilic addition in conjugated dienes gives a mixture of products. - with an isolated diene, electrophlic addition of one equivalent of HBr gives a single product and Markovnikov’s rule is followed (H adds to the less substituted carbon): Br HBr (1 equiv) - with conjugated dienes, electrophlic addition of one equivalent of HBr gives two products; 1,2-product from Markovnikov addition and 1,4product from conjugate addition: Br HBr (1 equiv) + Br 1,4-product 1,2- product Discuss mechanism – section 16.10 Thus, addition of HX to a conjugated diene forms 1,2- and 1,4-products because of the resonance stabilized allylic carbocation intermediate. In class problem: Draw products formed when each diene is treated with one equivalent of HCl. (a) (b) (b) H+ less reactive double bond ClCl HCl (1 equiv) more reactive double bond H+ Cl + Cl Cl- + Cl- likely a minor product 4 Kinetic versus thermodynamic products - the amount of 1,2- and 1,4-addition products formed in electrophilic addition reactions of conjugated dienes depends greatly on the reaction conditions: At low temperatures, the major product is formed by 1,2-addition. The kinetic product is formed faster and predominates at low temperature: Br HBr (1 equiv) + Br -80 ˚C 1,4-product 1,2- product 20% 80% At higher temperatures, the major product is formed by 1,4-addition. The more slowly formed thermodynamic product is more stable and predominates at higher temperatures Br HBr (1 equiv) 40 ˚C + Br 1,4-product 80% 1,2- product 20% In fact, when a mixture containing mainly the 1,2-product is heated, the 1-4-addition product becomes the major product at equilibrium: Br ! Br major product at low T major product at equilibrium Why is the 1,4-addition product the more stable (thermodynamic) product? Because more substituted alkenes are more stable (Remember, increasing alkyl substitution stabilizes an alkene by an electron-donating inductive effect) Why is the more stable conjugate addition product formed more slowly? Because higher activation energy is required for formation of the required intermediate allylic carbocation – Figure 16.6 and Fig 16.7 The 1,2-addition product is formed faster because of the proximity of Brto C2; Following H+ addition to the double bond, Br- is closer to C2 than C4 hence attack at C2 is faster. 5 Why is the product ratio temperature dependent? The activation energy is more important at low temperature where most molecules do not have enough kinetic energy to over the higher energy barrier. Hence they react by overcoming the lower energy barrier and form the kinetic product. Preparation of Conjugated Systems There are a number of methods for the preparation of conjugated systems. One possibility is by allylic bromination, followed by either conjugate (1) or normal (2) elimination: (1) KOBut NBS h! or ROOR Br DMSO + Br (2) Diels Alder Reaction One of the most spectacular reactions in organic chemistry. In linking two carbon molecules together, it forms two single bonds and one double bond, all in one step. At its simplest, a 1,3-diene reacts with an alkene (called a dienophile) in a concerted reaction to give a cyclohexene. 6 The Diels-Alder reaction is a thermal reaction, that is, it is initiated by heat. No intermediates of an ionic or radical nature have been detected for this reaction. It goes in one step (it is concerted). The reaction depicted above requires high temperatures and pressures in order to go. However, a Diels-Alder reaction can be performed at moderate temperature if a number of requirements are met. Several rules govern the course of a Diels-Alder reaction: 1. Electron withdrawing substituents in the dienophile increases the reaction rate. An electron-poor dienophile is required because the dienophile acts an electrophile in Diels-Alder reaction. Examples of good dienophiles include: - acrylonitrile, acrylic acid, acrolein, maleic anhydride, benzoquinone Clearly, use of an electron-rich diene is helpful since the diene acts a nucleophile in Diels-Alder reaction; e.g. OCH3 TMSO 2. The diene MUST be able to adopt an s-cis configuration; it can only react in this conformation 3. The stereochemistry of the dienophile is retained in the product. A cis dienophile forms a cis-substituted cyclohexene: 7 COOH COOH ! COOH or COOH COOH maleic acid COOH an achiral meso compound A trans dienophile forms a trans-substituted cyclohexene: HOOC COOH ! COOH + COOH COOH COOH fumaric acid enantiomers A cyclic dienophile forms a bicyclic cis -fused product: O H O H O ! O H H O O 4. When exo and endo products are possible, the endo product is preferred. e. g. H ! CH3 + H H CH3 O O H CH 3 H O H endo product is the major product exo product is the minor product O O H O O - H ! O O O + HO O H exo product is the endo product is the minor product major product in this type of reaction, we obtain a bridged bicyclic product in which the two rings share non-adjacent carbon atoms. The endo 8 product, in which the substituent(s) on the bridging carbon(s) end(s) up under the newly formed six-membered ring, is preferred. - To understand this preference, see Fig 16.11 – the transition state leading to the endo product allows more interaction between the electron rich diene and the electron withdrawing substituent on the dienophile; and this arrangement is energetically favorable. In class problem: draw the product of the following Diels Alder reaction COOCH3 ! + COOCH3 COOCH3 COOCH3 Regioselectivity in Diels-Alder Reactions – ortho-para rule When an unsymmetrical diene and an unsymmetrical dienophile combine in a Diels-Alder reaction, the simplest way to determine which product will be formed is to draw an “ionic” stepwise mechanism for the reaction to establish which end of the diene will react with which end of the dienophile. Reaction between a diene that is substituted at the terminal position with an electron donating group and a dienophile proceeds through an aromatic transition state that is ortho-directing and leads to 1,2substituted cycloehexene product; e.g. O O O OMe OMe OMe O OMe Note however, that the reaction is in fact concerted. Thus the transition state of the reaction really looks like below: O ‡ O O OMe OMe OMe 9 Reaction between a diene that is substituted at the internal position with an electron donating group and a dienophile proceeds through an aromatic transition state that is para-directing and leads to 1,4substituted cycloehexene product; e.g. O O O OMe MeO OMe OMe MeO MeO O OMe Actual mechanism MeO O OMe MeO O ‡ O OMe OMe MeO MeO Retrosynthetic analysis of a Diels-alder product: you should be able to look a Diels-Alder product and determine the conjugated diene and dienophile used to make it; e.g. H3CO COOCH3 COOCH3 H3CO + COOCH3 H3COOC Retro-Diels-Alder reaction Note also that in some cases the Diels-Alder reaction is reversible; i.e. a cyclohexene can revert back to a diene and a dienophile. 25˚ C H H ! Occasionally, the retro Diels-Alder is more favorable than the forward reaction. In these cases, there are some special tricks that can be used to 10 force the reaction along. One such case is the use of an orthoquinodimethane: - aromatic character of the product drives the reaction forward UV-Vis Spectroscopy. UV-Vis spectroscopy is based on exciting the electronic levels in conjugated molecules. What occurs is simply a promotion of one electron from the molecule’s HOMO into its LUMO. The molecule generally takes on the electronic character of the LUMO in this instance, generally having a diradical character. The greater the degree of conjugation in the molecule (i.e. the more levels in the M.O. picture), the easier it will be to excite an electron into the LUMO. At sufficiently low energy level differences, the energy required to promote an electron to the LUMO can be provided by visible light, yielding a colored compound. Another way to say this is that if a compund is colored, an easy route must exist for the promotion of an electron. Along with increasing the degree of conjugation, there are other ways to facilitate the excitation of an electron. One method is to facilitate what is called intramolecular charge transfer. This is usually accomplished by the preparation of a “push-pull” system, as shown below: The ease with which this charge-transfer reaction takes place depends on the strength of the “push” component (in this case, the dimethylamine group) and the strength of the “pull” component (the nitro group). Thus by varying the push and pull moieties, and by changing the length of the conjugated bridge separating them, we can control the color of the molecule! Some examples of colored compounds are shown below – be sure you understand why they have different colors! 11 12