Diastereoselective effects of the Diels-Alder

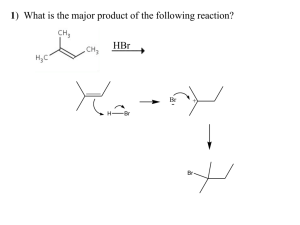
Synthetic efforts towards anthracyclines, using the
Asymmetric Diels-Alder Reaction
Doxorubicin, an effective drug used in chemotherapy (although other types of drugs are being developed and preferred now), has the 3-D structures below; notice in the first that the rings-A to D are nearly flat, but slightly buckled and the special half chair geometry of the ring-A. The next reveals the chair conformation of the daunosamine (sugar) auxiliary.
KEY: In all 3-D structures, atoms are not shown, however the bonds surrounding oxygen are red, the bonds surrounding carbon are grey, the bonds surrounding nitrogen are blue, the bond to hydrogen is light blue and the bonds around silicon are purple.
Crucial to the synthesis and a method of inducing the stereochemistry into the ring-A, is the Diels-Alder reaction, discovered by this manner in the Stoodley group,
Manchester, U.K. The D-glucose based diene ( as type 1 or 2 ), a modiefied
Danishefsky’s diene used the
-D-glucose moiety to control the stereochemistry of the cycloaddition; it’s 3-D structure is shown on the right. Notice the chair formation of the sugar group, which is it’s most stable form.
In all cases, the major cycloadduct arising from the cycloaddition is a result of bond formation to the less sterically hindered top face of the diene and there is secondary orbital overlap
1
between the HOMO of the diene and LUMO of the dienophile (i.e. the middle p-orbitals of the diene with the vacant p-orbitals of the carbonyl groups in
such a dienophile) in the transition state resulting in in endo adducts obtained under kinetically controlled conditions. The energy of this transition state is lower compared to the exo transition state.
The model above is based on the anomeric effect , to describe the conformation of the diene. An x-ray crystallographic study has shown that D-glucose based dienes of type
( 2 ) exists in the s-trans geometry ( 3 ). An NOED spectroscopic study by NMR in deuteriochloroform solution showed that mainly one conformer of type ( 3 ) was present. Presumably
2
, the steric interaction (green lines) between H x and H z in the conformer ( 4 ) outweighs that between H x and H y in conformer ( 3 ) and tips the balance in favour of the latter.
The adoption of conformations ( 3 ) and ( 4 ) is in accord with expectation based on the exo-anomeric effect, in which the O-1 (between the diene and sugar) is sp 2 hybridised and it’s p-orbital overlaps with the * antibonding orbital of the diene moiety and the
*
antibonding orbital as a result of the bond between C-1’ and O-1’.
My Diels-Alder reaction
In order to undergo a Diels-Alder reaction, the s-cis geometry of the diene must exist, as shown for ( 2 ) in the scheme above. The dienophile approaches the sterically lesshindered top face of conformer ( 2 ) to avoid an unfavourable syn -1,3-interaction between the C-1’-O-1’ bond in the sugar group. The major cycloadduct ( 7 ) is obtained in 36% experimental yield on a 1.8 mmol scale. The minor cycloadduct ( 8 ) arises from addition to either the top face of the unfavourable cis-conformer ( 4 ) or bottom
face of the preferred conformer ( 2 ). NMR spectroscopy of the mixture of cycloadducts before their separation by chromatography showed their ratio to be 3:1
(i.e. 60 % diastereomeric excess, d.e., ( 7 )] when the reaction had gone to completion.
Schemes to support the preparation of the dienophile ( 6 ) are detailed within the website.
REFERENCE
1. ‘Frontier Orbitals and Organic Chemical Reactions’, I. Fleming, p. 106, John Wiley
& Sons, Chichester, 1976. ISBN 0 471 01819 8.
2. R.C. Gupta, D.S. Larsen and R.J. Stoodley , J. Chem. Soc., Perkin Trans. 1 , 1989,
739.