Ch. 13 - Wiley
advertisement

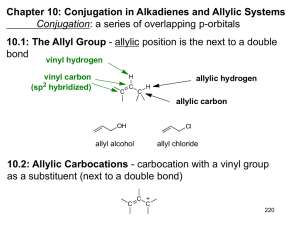
Chapter 13 Conjugated Unsaturated Systems Created by Professor William Tam & Dr. Phillis Chang Ch. 13 - 1 About The Authors These PowerPoint Lecture Slides were created and prepared by Professor William Tam and his wife, Dr. Phillis Chang. Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Guelph (Ontario, Canada) in 1998 and is currently a Full Professor and Associate Chair in the department. Professor Tam has received several awards in research and teaching, and according to Essential Science Indicators, he is currently ranked as the Top 1% most cited Chemists worldwide. He has published four books and over 80 scientific papers in top international journals such as J. Am. Chem. Soc., Angew. Chem., Org. Lett., and J. Org. Chem. Dr. Phillis Chang received her B.Sc. at New York University (USA) in 1994, her M.Sc. and Ph.D. in 1997 and 2001 at the University of Guelph (Canada). She lives in Guelph with her husband, William, and their son, Matthew. Ch. 13- 2 1. Introduction A conjugated system involves at least one atom with a p orbital adjacent to at least one p bond ● e.g. conjugated diene allylic radical allylic cation allylic anion O enone enyne Ch. 13 - 3 2. Allylic Substitution and the Allyl Radical vinylic carbons (sp2) X X2 low temp CCl4 X X2 allylic carbon (sp3) high temp (and low conc. of X2) + H X X Ch. 13 - 4 2A. Allylic Chlorination (High Temperature) + Cl2 400oC gas phase Cl + H Cl Ch. 13 - 5 Mechanism ● Chain initiation Cl Cl 2 Cl ● Chain propagation H + Cl + H (allylic radical) Cl Ch. 13 - 6 Mechanism ● Chain propagation + Cl Cl Cl + Cl ● Chain termination + Cl Cl Ch. 13 - 7 H + H DHo = 369 kJmol-1 H + H DHo = 465 kJmol-1 Ch. 13 - 8 H X + + H Relative stability of radicals: X Eact (low) Eact (high) o o + HX + HX o allylic > 3 > 2 > 1 > vinylic Ch. 13 - 9 Ch. 13 - 10 2B. Allylic Bromination with N-Bromosuccinimide (Low Concentration of Br2) NBS is a solid and nearly insoluble in CCl4 ● Low concentration of Br• Br H + O N O (NBS) h or ROOR heat, CCl4 H Br + O N O Ch. 13 - 11 Examples Br NBS ROOR, CCl4 heat NBS ROOR, CCl4 heat Br Ch. 13 - 12 3. The Stability of the Allyl Radical 3A. Molecular Orbital Description of the Allyl Radical Ch. 13 - 13 Ch. 13 - 14 3B. Resonance Description of the Allyl Radical 2 2 1 3 1 3 3 3 2 2 1 1 Ch. 13 - 15 4. The Allyl Cation Relative order of Carbocation stability > > (3o) (3o allylic) > > > (2o) (allylic) (1o) (vinylic) Ch. 13 - 16 5. Resonance Theory Revisited 5A. Rules for Writing Resonance Structures Resonance structures exist only on paper. Although they have no real existence of their own, resonance structures are useful because they allow us to describe molecules, radicals, and ions for which a single Lewis structure is inadequate We connect these structures by doubleheaded arrows (), and we say that the hybrid of all of them represents the real molecule, radical, or ion Ch. 13 - 17 In writing resonance structures, we are only allowed to move electrons resonance structures H H not resonance structures Ch. 13 - 18 All of the structures must be proper Lewis structures :O: X O 10 electrons! not a proper Lewis structure Ch. 13 - 19 All resonance structures must have the same number of unpaired electrons X Ch. 13 - 20 All atoms that are part of the delocalized p-electron system must lie in a plane or be nearly planar delocalization of p-electrons no delocalization of p-electrons Ch. 13 - 21 The energy of the actual molecule is lower than the energy that might be estimated for any contributing structure Equivalent resonance structures make equal contributions to the hybrid, and a system described by them has a large resonance stabilization Ch. 13 - 22 The more stable a structure is (when taken by itself), the greater is its contribution to the hybrid (3o allylic cation) (2o allylic cation) greater contribution Ch. 13 - 23 5B. Estimating the Relative Stability of Resonance Structures The more covalent bonds a structure has, the more stable it is (more stable) O (more stable) (less stable) O (less stable) Ch. 13 - 24 Structures in which all of the atoms have a complete valence shell of electrons (i.e., the noble gas structure) are especially stable and make large contributions to the hybrid O this carbon has 6 electrons O this carbon has 8 electrons Ch. 13 - 25 Charge separation decreases stability OMe (more stable) OMe (less stable) Ch. 13 - 26 6. Alkadienes and Polyunsaturated Hydrocarbons Alkadienes (“Dienes”) 4 2 1 2 3 1,3-Butadiene 4 2 1 6 1 3 3 5 4 6 5 1,3-Cyclohexadiene (2E,4E)-2,4-Hexadiene Ch. 13 - 27 Alkatrienes (“Trienes”) 4 2 1 3 6 5 8 7 (2E,4E,6E)-Octa-2,4,6-triene Ch. 13 - 28 Alkadiynes (“Diynes”) 1 2 4 3 5 6 2,4-Hexadiynes 6 Alkenynes (“Enynes”) 5 1 4 3 2 5 3 2 6 7 8 4 1 Hex-1-en-5-yne (2E)-Oct-2-en-6-yne Ch. 13 - 29 Cumulenes enantiomers H H C H C H H C C H H C C H (Allene) (a 1,2-diene) Ch. 13 - 30 Conjugated dienes Isolated double bonds Ch. 13 - 31 7. 1,3-Butadiene: Electron Delocalization 7A. Bond Lengths of 1,3-Butadiene 1.47 Å 4 2 1 3 1.34 Å sp3 1.54 Å sp2 sp3 1.50 Å sp sp3 1.46 Å Ch. 13 - 32 7B. Conformations of 1,3-Butadiene trans single bond single bond cis (s-cis) (s-trans) H H (less stable) Ch. 13 - 33 7C. Molecular Orbitals of 1,3-Butadiene Ch. 13 - 34 8. The Stability of Conjugated Dienes Conjugated alkadienes are thermodynamically more stable than isomeric isolated alkadienes H o (kJmol-1) 2 + 2 H2 2 2 x (-127)=-254 + 2 H2 =-239 Difference 15 Ch. 13 - 35 Ch. 13 - 36 9. Ultraviolet–Visible Spectroscopy The absorption of UV–Vis radiation is caused by transfer of energy from the radiation beam to electrons that can be excited to higher energy orbitals Ch. 13 - 37 9A. The Electromagnetic Spectrum Ch. 13 - 38 9B. UV–Vis Spectrophotometers Ch. 13 - 39 Ch. 13 - 40 Beer’s law A = excxℓ or e = A cxℓ A = e = c = ℓ = absorbance molar absorptivity concentration path length ● e.g. 2,5-Dimethyl-2,4-hexadiene lmax(methanol) 242.5 nm (e = 13,100) Ch. 13 - 41 9C. Absorption Maxima for Nonconjugated and Conjugated Dienes Ch. 13 - 42 Acetone O n Ground state p lmax = 280 nm emax = 15 O p* Excited state O n p lmax = 324 nm,emax = 24 p p lmax = 219 nm,emax = 3600 Ch. 13 - 43 9D. Analytical Uses of UV–Vis Spectroscopy UV–Vis spectroscopy can be used in the structure elucidation of organic molecules to indicate whether conjugation is present in a given sample A more widespread use of UV–Vis spectroscopy, however, has to do with determining the concentration of an unknown sample Quantitative analysis using UV–Vis spectroscopy is routinely used in biochemical studies to measure the rates of enzymatic reactions Ch. 13 - 44 10. Electrophilic Attack on Conjugated Dienes: 1,4 Addition 4 2 1 H Cl 25oC 3 Cl H (78%) (1,2-Addition) + H Cl (22%) (1,4-Addition) Ch. 13 - 45 Cl Mechanism H X H + H (a) Cl H (a) (a) Cl H (b) H + + (b) Cl H (b) Ch. 13 - 46 10A. Kinetic Control versus Thermodynamic Control of a Chemical Reaction -80oC Br + Br (20%) (80%) + HBr Br + 40oC (20%) Br (80%) Ch. 13 - 47 Br o 40 C, HBr Br 1,2-Addition product 1,4-Addition product Ch. 13 - 48 Ch. 13 - 49 11. The Diels–Alder Reaction: A 1,4-Cycloaddition Reaction of Dienes + (diene) (dienophile) [4p+2p] (adduct) Ch. 13 - 50 e.g. O + O O benzene O 100oC O 1,3-Butadiene Maleic (diene) anhydride (dienophile) O Adduct (100%) Ch. 13 - 51 11A. Factors Favoring the Diels–Alder Reaction Type A EDG EDG EWG EWG + EWG Type B EWG + EDG EDG ● Type A and Type B are normal Diels-Alder reactions Ch. 13 - 52 Type C EWG EWG EDG EDG + EDG Type D EDG + EWG EWG ● Type C and Type D are Inverse Demand Diels-Alder reactions Ch. 13 - 53 Relative rate O Diene + O 30oC D.A. cycloadduct O OMe > Diene t1/2 20 min. > 70 min. 4 h. Ch. 13 - 54 Relative rate 20oC + Dienophile NC CN NC CN Dienophile t1/2 0.002 sec. > D.A. cycloadduct CN > CN CN 20 min. 28 h. Ch. 13 - 55 Steric effects > Dienophile: COOEt Relative rate: 1 > COOEt 0.14 COOEt 0.007 Ch. 13 - 56 11B. Stereochemistry of the Diels–Alder Reaction 1. The Diels–Alder reaction is stereospecific: The reaction is a syn addition, and the configuration of the dienophile is retained in the product O H + H H O OMe OMe OMe OMe O Dimethyl maleate (a cis-dienophile) H O Dimethyl cyclohex-4-enecis-1,2-dicarboxylate Ch. 13 - 57 O + H H MeO O OMe OMe H O Dimethyl fumarate (a trans -dienophile) OMe H O Dimethyl cyclohex4-ene-trans -1,2dicarboxylate Ch. 13 - 58 2. The diene, of necessity, reacts in the s-cis rather than in the s-trans conformation s-cis Configuration s-trans Configuration O + R X O R Highly strained Ch. 13 - 59 e.g. COOMe + heat COOMe (diene locked in s-cis conformation) COOMe + (diene locked in s-trans conformation) heat No Reaction Ch. 13 - 60 Cyclic dienes in which the double bonds are held in the s-cis conformation are usually highly reactive in the Diels–Alder reaction Relative rate O Diene + O 30oC D.A. cycloadduct O > Diene t1/2 11 sec. > 130 sec. 4 h. Ch. 13 - 61 3. The Diels–Alder reaction occurs primarily in an endo rather than an exo fashion when the reaction is kinetically controlled H H R longest bridge H H R is exo H H R R is endo Ch. 13 - 62 Alder-Endo Rule ● If a dienophile contains activating groups with p bonds they will prefer an ENDO orientation in the transition state H H X X X X Ch. 13 - 63 e.g. + O O O H H 100% endo O O O Ch. 13 - 64 Stereospecific reaction (i) X X X X + X X + X X Ch. 13 - 65 Stereospecific reaction (ii) Y Y + Y Y Y Y Y + Y Ch. 13 - 66 (A) Examples Me Me NC CN + NC (B) CN CN D.A. CN CN CN Me Me NC CN Me + NC CN D.A. Me CN CN CN CN Ch. 13 - 67 Diene A reacts 103 times faster than diene B even though diene B has two electron-donating methyl groups Me Me Me Me H (s-cis) (s-trans) Ch. 13 - 68 Examples (C) O + O D.A. O H O (D) O + O O O H H D.A. O O O H O Ch. 13 - 69 Examples (E) O + O D.A. No Reaction O ● Rate of Diene C > Diene D (27 times), but Diene D >> Diene E ● In Diene C, tBu group electron donating group increase rate ● In Diene E, 2 tBu group steric effect, cannot adopt s-cis conformation Ch. 13 - 70 END OF CHAPTER 13 Ch. 13 - 71