conjugated diene - HCC Southeast Commons
advertisement

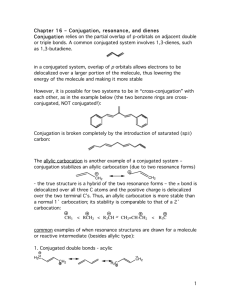
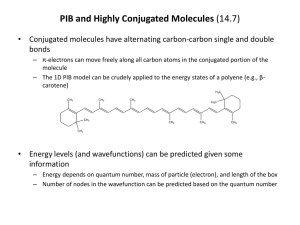
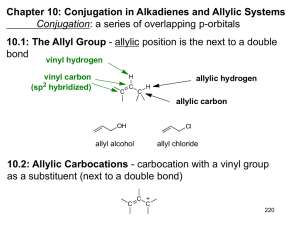
Chapter 14 Introduction • Compounds can have more than one double or triple bond. – Dienes are compounds with two double bonds – If they are separated by only one single bond, they are conjugated and their orbitals interact There are three types of dienes: • Conjugated: -C=C-C=C- alternating double and single bonds • Cumulated: -C=C=C- consecutive double bonds (no intervening single bond) • Isolated: -C=C-C-C=C- double bonds separated by more than one single bond (more than one intervening single bond) • Conjugated systems have distinctive properties. Example: The conjugated diene 1,3-butadiene has properties that are very different from those of the nonconjugated diene, 1,4-pentadiene • The term "conjugated" or "conjugated system" typically is applied to extended systems. • Polyenes - are compounds with many alternating single and double bonds - are conjugated hydrocarbons with many double bonds • Examples: a. beta-carotene/ vitamin A b. lycopene: the red pigment in tomatoes • Conjugated systems are common in nature and in biologically important molecules. enone (Alkene + Ketone) • Ultraviolet Spectroscopy (UV) – determines if a conjugated p electron system is present • One of the characteristics of conjugated systems is the absorbance of u.v. light by the p electron system. Red pigment 1. Preparation and Stability of Conjugated Dienes Conjugated dienes are generally prepared by: i. base-induced elimination of HX from an allylic halide ii. industrial catalytic dehydrogenation iii. industrial scale dehydration i. Base-induced elimination of HX from an allylic halide Example: Allylic bromination of Cyclohexene with NBS followed by elimination (tBOC) ii. Industrial catalytic dehydrogenation Example: 1,3- Butadiene, a substance used industrially to make polymers, is prepared by thermal cracking of butane in the presence of a catalyst (Chromium oxide/ aluminum oxide) iii. Industrial scale dehydration Example: Preparation of Isoprene via acid-catalyzed double dehydration of 3-Methyl-1,3-butanediol Bond Length • The central single bond in a conjugated diene is shorter than the single bond in a nonconjugated diene or an alkane. Example: The C2-C3 single bond in 1,3-butadiene is shorter than the C2-C3 bond in butane Stability • Conjugated dienes are more stable than nonconjugated dienes as evidenced by their heats of hydrogenation. • More highly substituted alkenes are more stable (release less heat of hydrogenation) than less substituted ones. Stability: 1,3-butadiene vs 1,4-pentadiene • Hydrogenating 1,3-butadiene releases 16 kJ/mol less heat than 1,4-pentadiene • Conjugation of the double bonds in 1,3-butadiene gives the extra stability of approximately 16 kJ/mol + 2 + • 2 H2 2 H2 cataly st 2 DHo = 2(-126 kJ/mol) = -252 kJ/mol) cataly st DHo = -236 kJ/mol) The unusual stability of 1,3-butadiene (and also other conjugated systems) is due to resonance energy (also called resonance stabilization or delocalization energy). Practice Problem: Allene, H2C=C=CH2, has a heat of hydrogenation of -298 kJ/mol (-71.3 kcal/mol). Rank a conjugated diene, a nonconjugated diene, and an allene in order of stability 2. Molecular Orbital Description of 1,3-Butadiene The unusual stability of conjugated dienes can be explained by: • Valence Bond Theory • Molecular Orbital Theory Valence Bond Theory • According to the valence bond theory, the stability of conjugated dienes is due to orbital hybridization: 25% s character 33% s character Electrons in sp2 orbitals are closer to the nucleus. Thus sp2-sp2 s bonds are shorter and stronger. Molecular Orbital Theory • According to the molecular orbital theory, the stability of conjugated dienes is due to interaction between the p orbitals of the two double bonds: – The bonding p-orbitals are made from 4 p orbitals that provide greater delocalization and lower energy than in isolated C=C – The single bond between the two double bonds is strengthened by overlap of p orbitals • Two p orbitals combine to form two p molecular orbitals: Bonding and Antibonding • Both electrons occupy the low energy, bonding orbital, forming a stable bond. higher in energy lower in energy • In a conjugated diene, four adjacent p orbitals combine to form four p molecular orbitals: two bonding and two antibonding fully additive • The four p electrons occupy the two bonding orbitals The number of nodes between nuclei increases as the energy level of the orbital increases p Molecular Orbitals: 1,3-butadiene vs 1,4-pentadiene • In a conjugated diene (1,3-butadiene), the lowest-energy p MO (y1) has a favorable bonding interaction between C2 and C3 that is absent in a nonconjugated diene – C2-C3 bond has partial double-bond character – C2-C3 bond is stronger and shorter than a typical single bond In a conjugated diene, the p electrons are delocalized or spread out over the entire p framework rather than localized between two specific nuclei. • • Electron delocalization always leads to greater stability. • Systems containing conjugated double bonds, not just those of dienes, are more stable than those containing nonconjugated double bonds. O O 2-Cyclohexenone (more stable) 3-Cyclohexenone (less stable) 3. Electrophilic Additions to Conjugated Dienes: Allylic Carbocations • Conjugated dienes undergo electrophilic addition reactions via a different mechanism than that observed in nonconjugated dienes: 1,4-addition General Mechanism of electrophilic addition reaction • Attack on electrophile (such as HX) by a p bond of alkene (nucleophile) • Formation of carbocation and halide ion • Reaction of nucleophilic halide ion with carbocation (an electrophile) Markovnikov’s Regiochemistry In the addition of HX to alkene: – The H attaches to the carbon with the most H’s and X attaches to the carbon with the most alkyl substituents – The more highly substituted (more stable) carbocation is formed as the intermediate rather than the less highly substituted one Electrophilic additions: Alkenes and Nonconjugated Dienes • Alkenes and nonconjugated dienes give Markovnikov’s products Electrophilic additions: Conjugated Dienes • Conjugated dienes give mixtures of products: 1,2 adduct and 1,4 adduct 1,2 adduct 1,4 adduct Constitutional isomers • Conjugated dienes give mixtures of products: 1,2 adduct and 1,4 adduct 1,4 adduct 1,2 adduct Constitutional isomers Carbocations from Conjugated Dienes Two possible carbocations of electrophilic addition to conjugated dienes: – secondary allylic carbocation – primary nonallylic carbocation (NOT formed) • The key intermediate formed is the delocalized secondary allylic carbocation because: – it is more stable, stabilized by resonance between two forms – it forms faster than a nonallylic carbocation Products of Addition to Delocalized Carbocation • Nucleophile can add to either cationic site: – 1,2 and 1,4 addition products result – The transition states for the two possible products are not equal in energy Practice Problem: Give the structures of the likely products (both 1,2 adducts and 1,4 adducts) for this reaction Practice Problem: Give the structures of both 1,2- adducts and 1,4 adducts resulting from reaction of 1 equivalent of HCl with 1,3-pentadiene. Practice Problem: Look at the possible carbocation intermediates produced during addition of HCl to 1,3pentadiene, and predict which 1,2 adduct predominates. Which 1,4 adduct predominates? Practice Problem: Give the structures of both 1,2 and 1,4 adducts resulting from reaction of 1 equivalent of HBr with the following substance: 4. Kinetic vs Thermodynamic Control of Reactions Electrophilic addition to a conjugated diene leads to a mixture of 1,2 and 1,4 addition products in varying amounts depending on the reaction conditions: • 1,2 adduct is usually formed faster and is said to be the product of kinetic control • 1,4 adduct is usually more stable and is said to be the product of thermodynamic control Example: The addition reaction of HBr to 1,3-butadiene has unusual temperature dependence – At low temperatures (0° C), the 1,2-addition products predominate – At higher temperatures (40°C), the 1,4-addition products predominate A reaction energy diagram for two competing reactions • B forms faster than C: DG‡B < DG‡C • C is more stable than B: DGoC > DGoB Kinetic Control • Under kinetic control, the product of an irreversible reaction depends on relative rates of formation. – If a reaction is irreversible or far from equilibrium, then the relative concentrations of products depend on how fast each forms, which is controlled by the relative free energies of the transition states leading to each (Kinetic Control) Thermodynamic Control • Under thermodynamic control, the product of a readily reversible reaction depends on thermodynamic stability – At completion, all reactions are at equilibrium and the relative concentrations are controlled by the differences in free energies of reactants and products (Thermodynamic Control) Example: Electrophilic Addition of HBr to 1,3-butadiene • Under kinetic control (at lower temperatures), there is a limited amount of energy available, sufficient only to overcome the lowest activation energy barrier, which leads to the 1,2-addition product. • Under thermodynamic control (higher temperatures), there is enough energy to overcome the larger activation energy barrier, which leads to the 1,4 adduct. • If the temperature is high enough, both reaction can reach equilibrium, in which case the more stable product (1,4-addition according to Zaitsev's rule) will predominate. Practice Problem: The 1,2 adduct and the 1,4 adduct formed by reaction of HBr with 1,3-butadiene are in equilibrium at 40oC. Propose a mechanism by which the interconversion of products takes place. Practice Problem: Why do you suppose 1,4 adducts of 1,3butadiene are generally more stable than 1,2 adducts? 5. The Diels-Alder Cycloaddition Reaction The Diels-Alder cycloaddition reaction is unique to conjugated dienes. • Conjugated dienes can combine with alkenes to form six-membered cyclic compounds • Example: The Diels-Alder reaction – is a cycloaddition reaction, i.e one in which two reactants add together in a single step to form a cyclic product – was discovered by Otto Paul Hermann Diels and Kurt Alder in Germany in the 1930’s (awarded the 1950 Nobel Prize) The Diels-Alder cycloaddition reaction – forms two C-C bonds in one step. – is one of only a few ring-forming reactions. – is said to be "pericyclic," not polar or free-radical (Woodward and Hoffman in 1965) – is a single, one-step process with no intermediates (concerted formation of two bonds) The Diels-Alder cycloaddition – involves orbital overlap, change of hybridization and electron delocalization in transition state sp2 sp2 sp3 sp2 sp2 sp2 sp2 Head on (s) overlap of two alkene p orbitals with two p orbitals on C1 and C4 of the diene sp3 6. Characteristics of the Diels-Alder Reaction The Diels-Alder cycloaddition – – involves a dienophile and a diene is stereospecific and regioselective a diene a dienophile Diels-Alder adduct The Dienophile – is “diene-loving” – has an alkene (C=C) or alkyne (CC) component conjugated to an electron-withdrawing group, such as C=O or CN – has a double (C=C) or triple (CC) bond next to the positively polarized carbon of an electronwithdrawing substituent The Dienophile – has an alkene (C=C) or alkyne (CC) component conjugated to an electron-withdrawing group, such as C=O or CN The Dienophile – has a double (C=C) or triple (CC) bond next to the positively polarized carbon of an electron-withdrawing group The electron-withdrawing group makes the double bond carbons less negative Stereospecificity of the Diels-Alder Reaction • The Diels-Alder reaction is stereospecific: – It maintains relative relationships from reactant to product – There is a one-to-one relationship between stereoisomeric reactants and products • The Diels-Alder reaction is stereospecific: – The two carbons of the dienophile add to the same face of the diene. – The stereochemistry of the dienophile is maintained, and a single product stereoisomer results cis dienophile reactant gives cis-substituted cyclohexene product Cis dienophile reactant gives cis-substituted cyclohexene product Trans dienophile reactant gives trans-substituted cyclohexene product Regiospecificity of the Diels-Alder Reaction • The Diels-Alder reaction is regiospecific: – The diene and dienophile reactants align to produce endo (rather than exo) product Endo and exo are relative to the double bond derived from the diene • Endo and exo indicate relative stereochemistry in bicyclic structures • Substituent on one bridge is: – exo if it is anti (trans) to the larger of the other two bridges – endo if it is syn (cis) to the larger of the other two bridges • Endo products are formed because orbital overlap increases when the diene and dienophile reactants align so that the electron-withdrawing group of the dienophile is underneath the diene. Practice Problem: Predict the product of the following DielsAlder reaction: Practice Problem: Predict the product of the following DielsAlder reaction: The Diene – must have the s-cis conformation (“cis-like” about the single bond) to undergo the Diels-Alder reaction (higher in energy) (lower in energy) The Diene – must have the s-cis conformation because only in the s-cis conformation are C1 and C4 of the diene close enough to react through a cyclic transition state (overlap with dienophile p orbitals) – Dienes that cannot adopt the s-cis conformation are unreactive in the Diels-Alder reaction – Examples – Other dienes that are fixed in the s-cis conformation are highly reactive in the Diels-Alder reaction – Example: Dimerization of 1,3-cyclopentadiene The Diels-Alder cycloaddition – is facilitated by a combination of electron-withdrawing substituents on one reactant and electron-releasing substituents on the other – Example: a diene a dienophile Diels-Alder adduct The Diels-Alder cycloaddition – is facilitated by a combination of electron-withdrawing substituents on one reactant and electron-releasing substituents on the other Electron-releasing Group -CH3, alkyl groups -OR (ether) -OOCR (ester) Electron-withdrawing Group -CN (cyano) -CHO (aldehyde, ketone) -COOH (carboxyl) -COOR (ester) -NO2 (nitro) Practice Problem: Which of the following alkenes would you expect to be good Diels-Alder dienophiles? Practice Problem: Which of the following dienes have an s-cis conformation, and which have an s-trans conformation? Of the s-trans dienes, which can readily rotate to s-cis? Practice Problem: Predict the product of the following DielsAlder reaction: 7. Diene Polymers: Natural and Synthetic Rubbers • Conjugated dienes can be polymerized cis trans • Polymerization: is 1,4 addition of growing chain to conjugated diene monomer • The initiator for the reaction can be: – a radical or – an acid Natural Rubber • Two naturally occurring polymers of isoprene are: – natural rubber (Z isomer) – gutta-percha (E isomer) • The repeating unit has 5 carbons head-to-tail polymer of isoprene Synthetic Rubber • Synthetic rubbers are produced commercially by diene polymerization • Example: Neoprene (a polymer of chloroprene) Synthetic rubber (weather resistant) Vulcanization • Natural and synthetic rubbers are too soft to be used in products unless hardened by vulcanization • Vulcanization – was discovered by Charles Goodyear – involves heating the crude polymer with small amount of sulfur to produce strong material • Sulfur forms bridges between hydrocarbon chains (cross-links), locking the chains Practice Problem: Draw a segment of the polymer that might be prepared from 2-phenyl-1,3-butadiene. Practice Problem: Show the mechanism of the acid-catalyzed polymerization of 1,3-butadiene. 8. Structure Determination in Conjugated Systems: Ultraviolet Spectroscopy • Mass Spectrometry (MS) – determines the size and formula • Infrared (IR) Spectroscopy – determines the kinds of functional groups present • Nuclear Magnetic Resonance Spectroscopy (NMR) – – determines the carbonhydrogen framework • Ultraviolet Spectroscopy (UV) – determines if a conjugated p electron system is present • The ultraviolet (UV) region is higher in photon energy than visible light – The region from 200 to 400 nm (2 x 10-7 m to 4 x 10-7 m) is most useful in organic chemistry • Conjugated compounds can absorb light in the ultraviolet region of the spectrum – The energy absorbed corresponds to the amount necessary to promote an electron from one orbital to another – The electrons in the highest occupied molecular orbital (HOMO) undergo a transition to the lowest unoccupied molecular orbital (LUMO) Practice Problem: Calculate the energy range of electromagnetic radiation in the UV region of the spectrum from 200 to 400 nm. Recall the equation E = NA e = NA hc l = 1.20 x 10-4 kJ/mol l Practice Problem: How does the energy you calculated in the previous problem for UV radiation compare with the values calculated previously for IR and NMR spectroscopy? 9. Ultraviolet Spectrum of 1,3Butadiene • 1,3-butadiene has four p molecular orbitals with the two lower-energy MOs occupied (y1 and y2) • When 1,3-butadiene absorbs UV light, a p electron in the highest occupied molecular orbital (HOMO) is “promoted” to the lowest unoccupied molecular orbital (LUMO). – This corresponds to a p p* excitation – This transition requires 217 nm UV light (lmax) • A UV spectrum is a plot of absorbance versus wavelength. – A UV spectrum of purified molecule is obtained by irradiating a sample with a linearly changing wavelength of UV light and measuring the amount of light absorbed at each wavelength. A = log Io I • The Beer-Lambert Law gives: A=exCxl where A = Absorbance = log(% of light transmitted through the sample) e = molar absorptivity (extinction coefficient) in M-1cm-1 C = concentration in mol/L l = pathlength in cm • Absorbance for a particular compound in a specific solvent at a specified wavelength is directly proportional to its concentration Practice Problem: If a pure vitamin A sample has an absorbance at 325 nm of 0.735 in a 1.00 cm cell and e is known to be 50,100 M-1 cm-1. What is its concentration? A=exCxl Rearranging the equation, C= A exl 0.735 C= 50,100 M-1 cm-1 x 1.00 cm C = 1.47 x 10-5 M 10. Interpreting Ultraviolet Spectra: The Effect of Conjugation • tlmax is the wavelength where UV absorbance for a compound is greatest • It depends on: – the energy difference between HOMO and LUMO – the extent of conjugation • llmax increases as conjugation increases (lower energy) – Energy difference between HOMO and LUMO decreases as the extent of conjugation increases Molecule 1,3-butadiene 1,3,5-hexatriene 1,3,5,7-octatetraene lmax (nm) 217 nm 258 nm 290 nm • Substituents on p system increase lmax Practice Problem: Which of the following compounds would you expect to show ultraviolet absorptions in the 200 to 400 nm range? 11. Conjugation, Color, and the Chemistry of Vision • The visible region is about 400 to 800 nm, adjacent to the UV region. • Extended systems of conjugation absorb in visible region; they are colored • Example: b-Carotene, 11 double bonds in conjugation, lmax = 455 nm • Example: b-Carotene is yellow-orange; it absorbs the blue wavelength and transmits the rest 380 450 500 550 600 650 700 750 • Light-sensitive molecules responsible for vision are conjugated systems. (dietary) (in liver) • Visual pigments are responsible for absorbing light in eye and triggering nerves to send signal to brain trans-rhodopsin found in rod cells (lightsensitive receptor cells responsible for dim light vision) sends signal to brain Chapter 14