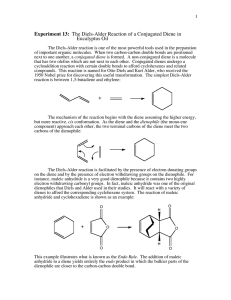
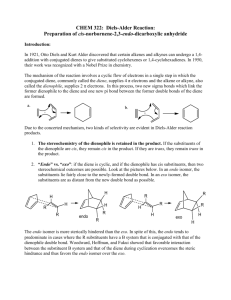
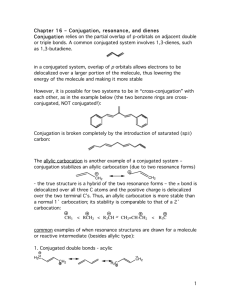
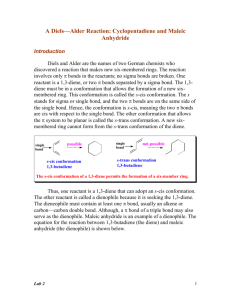
The Diels-Alder Reaction of a Conjugated Diene in
advertisement

CH242L W2008 Maazouz PCC-Sylvania Exp #3 The Diels-Alder Reaction of a Conjugated Diene in Eucalyptus Oil Pre-Lab Read Bruice Chapter 7.12, Dienes, p. 326-333 Complete items numbered 1-6 as described in your lab syllabus. Make sure your data table includes the appropriate physical properties (chemical structure, molecular weight, densities, boiling points for liquids, and purpose) for ALL the chemicals used in this lab. Print a copy of the FTIR reference spectra for all four possible products and maleic anhydride (you will need to use the wild card to obtain the spectra from the SDBS website i.e. %terpinene) and staple them to your pre-lab. Remember to write in pen in your lab notebook, and to turn in your carbon copies before you start the lab. START YOUR PROCEDURE AND OBSERVATIONS ON A SEPARATE PAGE. Additional Pre-lab questions to be turned in at the start of lab. 1. Compare your four reference IR spectra. What distinguishing features will allow you to identify your product? 2. What are the importantant characteristics of a good dienophile? 3. What is the difference between an endo and an exo product? 4. How is a solid sample prepared as a nujol mull? 5. Using the Gas Chromatograph for the Eucalyptus Oil located at the end of this handout, estimate the mass of the unknown diene present in 0.5 g of eucalyptus oil. Assume that the unknown conjugated diene corresponds to the largest peak on the chromatogram and that the peak areas are proportional to the component masses. Calculate the mass of maleic anhydride needed to react with that much diene. Notice that all the unknown dienes have the same molecular weight. Introduction Otto Diels and Kurt Alder received the 1950 Nobel Prize for discovering one of the most powerful tools used in the preparation of important organic molecules now called The Diels-Alder Reaction. When two carbon-carbon double bonds are positioned next to one another, a conjugated diene is formed. A non-conjugated diene is a molecule that has two olefins that are not next to each other. Conjugated dienes undergo a cycloaddition reaction with certain double bonds to afford cyclohexenes and related compounds. The simplest Diels-Alder reaction is between 1,3-butadiene and ethylene to produce cyclohexene. + diene dienophile The mechanism of the reaction, also known as a “4 + 2 cycloaddition”, begins with the diene assuming the higher energy, but more reactive, cis conformation. As the diene and the dienophile (the mono-ene component) approach each other, the two terminal carbons of the diene meet the two carbons of the dienophile. The Diels-Alder reaction is facilitated by the presence of electron donating groups on the diene and by the presence of electron withdrawing groups on the dienophile. For instance, maleic anhydride is a very good dienophile because it contains two highly electron withdrawing carbonyl groups. In fact, maleic anhydride was one of the original dienophiles that Diels and Alder used in their studies. It will react with a variety of dienes to afford the corresponding cyclohexene system. The reaction of maleic anhydride and 1,3-butadiene is shown as an example: CH242L W2008 Maazouz PCC-Sylvania O + O O O O O This example illustrates what is known as the Endo Rule. The addition of maleic anhydride to a diene yields entirely the endo product in which the bulkier parts of the dienophile are closer to the carbon-carbon double bond. Dienes and trienes occur in the essential oils of a number of plants and contribute to their flavors and aromas. You will separate the conjugated diene that is present in eucalyptus oil by reacting it with maleic anhydride. The unknown diene will be one of the four following conjugated dienes shown below: In order to determine the amount of maleic anhydride which will be necessary to react with all of the conjugated diene present, you will estimate the percentage of diene found in eucalyptus oil using the gas chromatogram. Since both maleic anhydride and the product of the Diels-Alder reaction can be hydrolyzed by water, it is important to use dry glassware and to exclude moisture during the reaction and throughout the procedure. The melting point of the product will reveal the identity of the conjugated diene in the oil. In addition, you will characterize the product by obtaining an infrared spectrum with the solid product in a Nujol mull. Compare your spectrum to that of maleic anhydride itself. CH242L W2008 Maazouz PCC-Sylvania Procedure In a clean and dry 5 mL round bottom flask, dissolve 0.5 g (weigh to 0.001 g) of the eucalyptus oil in 1 mL of anhydrous diethyl ether and add the amount of maleic anhydride, which you calculated in the pre-lab. Caution: Diethyl ether is highly flammable! Maleic anhydride is corrosive and toxic. Set up a reflux apparatus and gently reflux the mixture on a steam bath for 45 minutes. While it is warm, transfer the reaction mixture to a small beaker, cover with a watch glass, and let cool to room temperature. Cool it further on ice before collecting the crystals by vacuum filtration. Wash the crystals with 1 mL of cold petroleum ether. You may choose to recrystallize the Diels-Alder product from methanol, but I would recommend that you obtain a yield, MP, and FTIR prior to this step.. Heat the methanol on a steam bath. Important! Methanol can cause the product to undergo solvolysis, so it is important that you avoid prolonged boiling during recrystallization. Measure the melting point of the product so that you can identify which of the four possible dienes is present in eucalyptus oil. Leave the solid in your drawer for one week and re-measure the melting point when it is dry. Weigh the dry product next week. Grind a small amount (spatula-tip full, 20+ mg) of the product on a watch glass with the end of a stirring rod until it has a glassy appearance. Add 2-3 drops of Nujol to the watch glass and grind the mixture until it has the consistency of Vaseline. Using a rubber policeman, transfer some of the mixture to a salt plate. Spread the mull evenly over the surface of the plate using the second plate. Be especially careful to exclude air bubbles. Run the spectrum. A spectrum of pure Nujol is taped to the IR spectrometer. Wash your salt plates with a little acetone when finished to clean them! Be extremely careful not to drop the salt plates! Calculate the percent yield of the Diels-Alder product based on the mass of maleic anhydride used. Attach your IR spectrum. Identify which peaks in your spectrum arise from Nujol and which arise from your product. You should be able to identify the two bands which arise from the carbonyl stretching modes. Identify the diene found in eucalyptus oil. Write the correct structure of your Diels-Alder product you believe was formed in the reaction. Questions: 1. Write balanced equations for two of the four possible dienes. 2. Identify the sources in nature for each of the four possible dienes.