Ch. 14 & 15 Topics and Tips
advertisement

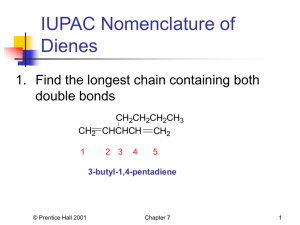
Leader: Hannah J. Course: Chem 331 Supplemental Instruction Instructor: Dr. Winter Iowa State University Date: 12/4/15 The following is a (checkable) list of the topics from Ch. 14 and 15 you should know and understand for Exam 4 and the Final Exam. Ch. 14 & 15 Topics and Tips Ch. 14: Conjugated Alkenes Definition of conjugation and identifying conjugated and non-conjugated alkenes Know that conjugated are more stable than non-conjugated and why (as it relates to resonance and heat released by each) Reaction & Mechanism: Reactions of Dienes with HX o 1,2-addition and 1,4-addition and their products (mixture) o Kinetic and Thermodynamic Products and which is present at high and low temperatures o How this all relates to an energy diagram of 1,2- and 1,4-addition Diels-Alder Reaction & Mechanism o Identifying diene and dienophile, the meaning of each name, and which should be electron-rich and electron-poor (nucleophile or electrophile) for the best DielsAlder reaction o Know examples of electron-donating and electron-withdrawing groups and that EDG (electron-donating groups) go on dienes and EWG (electron-withdrawing groups) go on dienophiles and why (in terms of electron-rich/poor) o The two traits that contribute to a group’s overall electron-donating or electronwithdrawing characteristic (electronegativity (i.e. the inductive effect) and resonance) and how they contribute or take away electron density in EDG and EWG o Know that dienes react for Diels-Alder reactions only in the s-cis conformation (Cycle dienes are the best because they are “locked” in this conformation; in straight-chain dienes, only a small fraction will be in the reactive s-cis conformation at any given moment because it is less stable than s-trans) o The Diels-Alder reaction always forms a 6-membered ring with a double bond (a cyclohexene). This double bond will always be between C’s 2 & 3 if you use the numbering system from Dr. Winter’s lectures. Anything else you see in the product is simply a substituent off of this ring and should also appear somewhere in your reactants. o Bicyclic rings are simply a fusing of two rings; they are only formed when the diene is cyclic (in a ring). These are represented as a 1- or 2-C bridge above the normal cyclohexene connected at C1 and C4 (in Dr. Winter’s numbering system). Think of them as another substituent to the basic ring. o Stereochemistry of Diels-Alder reactions and the Endo Rule: Know the conditions for which the Endo Rule applies and how the stereochemistry should look when the Endo Rule does or does not apply o Don’t be thrown by the 3-D representations of the product; these are interchangeable with their flat counterparts. You could be given either on an exam 1060 Hixson-Lied Student Success Center 515-294-6624 sistaff@iastate.edu http://www.si.iastate.edu and asked to fill in the reactants, but if you are asked to fill in the product(s), you can choose if you would rather give it/them in the flat or 3-D version. o There are a number of ways that Diels-Alder reactions can vary. Practice problems with triple bonds, multiple equivalents of a reactant, filling in either the reactants or the products, etc., and make sure that you know the mechanism and your numbering system well so that you can predict the missing pieces in a new variation. Ch. 15: Aromatics Memorize the common names and the corresponding structures of the seven common aromatics given in class 12/2/15. Nomenclature: o Know the 3 positioning names (ortho, meta, and para) and which corresponds to which position. In most cases, numbering is not needed for aromatics because these positioning names are used instead. o Practice giving missing IUPAC names or structures for aromatic compounds. Get comfortable combining the positioning and 7 memorized common names with the substituent names we’ve learned in past units into a single name or structure. Not all rings with alternating double bonds are aromatic! Know the requirements for aromaticity and anti-aromaticity and the special stability or instability of each type. Practice identifying molecules as aromatic, anti-aromatic, or neither. o Exception to normal sp3 hybridization rules: a lone pair next to a double bond will re-hybridize to sp2 (for increased stability of conjugation) o Know what to do when heteroatoms (O, N, S) with lone pairs show up in the structure: If the heteroatom is part of a double bond, the pi orbitals are already used, so the lone pairs are not in the pi system. If the heteroatom is not part of a double bond, it can contribute a maximum of one lone pair to the pi system (to your count of pi electrons in requirement 4). o Be familiar with how charges affect a structure’s aromaticity in terms of hybridization and/or pi electrons (for requirements 3 & 4)