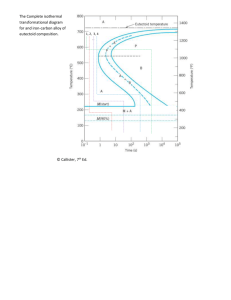
CHAPTER 10- PHASE TRANSFORMATIONS IN METALS PROBLEM SOLUTIONS 10.6 For this problem, we are given, for the recrystallization of aluminum, two values of y and two values of the corresponding times, and are asked to determine the fraction recrystallized after a total time of 116.8 min. The first thing necessary is to set up two expressions of the form of Equation (10.1), and then to solve simultaneously for the values of n and k. In order to expedite this process, we will rearrange and do some algebraic manipulation of Equation (10.1). First of all, we rearrange as follows: ( n) 1 − y = exp −kt Now taking natural logarithms ln (1 − y ) = − ktn − ln (1 − y) = ktn which may also be expressed as 1 n ln = kt 1 − y Now taking natural logarithms again, leads to 1 ln ln = ln k + n ln t 1 − y which is the form of the equation that we will now use. The two equations are thus 1 ln ln = ln k + n ln (95.2 min) 1 − 0.30 1 ln ln = ln k + n ln (126.6 min) 1 − 0.80 Solving these two expressions simultaneously for n and k yields n = 5.286 and k = 1.239 x 10-11. Now it becomes necessary to solve for y when t = 116.8 min. Application of Equation (10.1) leads to ( n) y = 1 − exp −kt ( ) 5.286 = 1 − exp − 1.239 x 10-11 (116.8 min) = 0.65 10.11 We are called upon to consider the isothermal transformation of an iron-carbon alloy of eutectoid composition. (a) From Figure 10.13, a horizontal line at 550°C intersects the 50% and reaction completion curves at about 2.5 and 6 seconds, respectively; these are the times asked for in the problem. 278 (b) The pearlite formed will be fine pearlite. From Figure 10.21(a), the hardness of an alloy of composition 0.76 wt% C that consists of fine pearlite is about 265 HB (27 HRC). 10.14 This problem asks us to determine the nature of the final microstructure of an iron-carbon alloy of eutectoid composition, that has been subjected to various isothermal heat treatments. Figure 10.13 is used in these determinations. (a) 50% coarse pearlite and 50% martensite (b) 100% spheroidite (c) 50% fine pearlite, 25% bainite , and 25% martensite (e) 40% bainite and 60% martensite (g) 100% fine pearlite (d) 100% martensite (f) 100% bainite (h) 100% tempered martensite 10.18 Below is shown an isothermal transformation diagram for a 1.13 wt% C iron-carbon alloy, with time-temperature paths that will produce (a) 6.2% proeutectoid cementite and 93.8% coarse pearlite; (b) 50% fine pearlite and 50% bainite; (c) 100% martensite; and (d) 100% tempered martensite. 10.20 Below is shown a continuous cooling transformation diagram for a 0.35 wt% C iron-carbon alloy, with continuous cooling paths that will produce (a) fine pearlite and proeutectoid ferrite; 279 (b) martensite; (c) martensite and proeutectoid ferrite; (d) coarse pearlite and proeutectoid ferrite; and (e) martensite, fine pearlite, and proeutectoid ferrite. 10.29 Two reasons why martensite is so hard and brittle are: 1) there are relatively few operable slip systems for the body-centered tetragonal crystal structure, and 2) virtually all of the carbon is in solid solution, which produces a solid-solution hardening effect. 10.31 This question asks for an explanation as to why the hardness of tempered martensite diminishes with tempering time (at constant temperature) and with increasing temperature (at constant tempering time). The hardness of tempered martensite depends on the ferrite-cementite phase boundary area; since these phase boundaries are barriers to dislocation motion, the greater the area the harder the alloy. The microstructure of tempered martensite consists of small sphere-like particles of cementite embedded within a ferrite matrix. As the size of the cementite particles increases, the phase boundary area diminishes, and the alloy becomes softer. Therefore, with increasing tempering time, the cementite particles grow, the phase boundary area decreases, and the hardness diminishes. As the tempering temperature is increased, the rate of cementite particle growth also increases, and the alloy softens, again, because of the decrease in phase boundary area. 10.32 In this problem we are asked to describe the simplest heat treatment that would be required to convert a eutectoid steel from one microstructure to another. Figure 10.18 is used to solve the several parts of this problem. (a) For martensite to spheroidite, heat to a temperature in the vicinity of 700°C (but below the eutectoid temperature), for on the order of 24 h. (b) For spheroiridte to martensite, austenitize at a temperature of about 760°C, then quench to room temperature at a rate greater than about 140°C/s. (c) For bainite to pearlite, first austenitize at a temperature of about 760°C, then cool to room temperature at a rate less than about 35°C/s. (d) For pearlite to bainite, first austenitize at a temperature of about 760°C, rapidly cool to a temperature between about 220°C and 540°C, and hold at this temperature for the time necessary to complete the bainite transformation (according to Figure 10.13). (e) For spheroidite to pearlite, same as (c) above. (f) For pearlite to spheroidite, heat at about 700°C for approximately 20 h. (g) For tempered martensite to martensite, first austenitize at a temperature of about 760°C, and rapidly quench to room temperature at a rate greater than about 140°C/s. (h) For bainite to spheroidite, simply heat at about 700°C for approximately 20 h. 280 10.D2 This problem asks if it is possible to produce an iron-carbon alloy that has a minimum tensile strength of 620 MPa (90,000 psi) and a minimum ductility of 50%RA. If such an alloy is possible, its composition and microstructure are to be stipulated. From Equation (6.20a), this tensile strength corresponds to a Brinell hardness of HB = TS(MPa) 620 MPa = = 180 3.45 3.45 According to Figures 10.21(a) and (b), the following is a tabulation of the composition ranges for fine and coarse pearlites and spheroidite which meet the stipulated criteria. Compositions for Compositions for Microstructure HB ≥ 180 %RA ≥ 50% Fine pearlite > 0.38 %C < 0.36 %C Coarse pearlite > 0.47 %C < 0.42 %C Spheroidite > 0.80 %C 0-1.0 %C Therefore, only spheroidite has a composition range overlap for both of the hardness and ductility restrictions; the spheroidite would necessarily have to have a carbon content greater than 0.80 wt% C. 281