Phase Transitions in Metal
advertisement

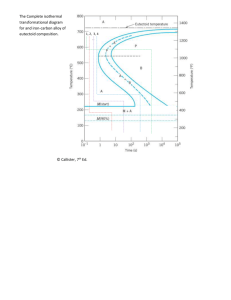
Materials Science 03/06/16 1/4 Lecture 10 Phase Transitions in Metals We previously looked at equilibrium phase diagrams, but curiously ignored dependence on time (for the most part). Kinetics (the time spent at elevated temperature, rate of temperature change, etc.) are also important. As mentioned earlier, it is possible to lock in non-equilibrium microstructures, or phases. These transformation are often limited by diffusion rates. What is a phase transformation, you ask? Remember the eutectoid reaction … The fraction of the transformation completed is given by the Avrami equation The rate of transformation is defined by convention as Two processes are involved in phase transformations: 1) Nucleation - formation of small particles of new phase 2) Growth - nuclei increase in size Materials Science 03/06/16 2/4 Lecture 10 Typically, this is plotted as follows (see fig 10.2) Back to the eutectoid reaction: Near the eutectoid temperature, this two phase mixture is called pearlite (b/c it looks like mother of pearl in a microscope). Pearlite is a mix of the soft ductile alpha-ferrite and the hard brittle iron carbide (cementite). Hypoeutectoid -Hypereutectoid – There are different microstructures in each case. We will examine the eutectoid case heavily. The information we want can be plotted as but this is too bulky. Materials Science 03/06/16 3/4 Lecture 10 Isothermal Transformation Diagrams Also called T-T-T plots – time temperature-transformation plots. Sketch the T-T-T plot for steel at the eutectoid (i.e. austenite to pearlite). Limitation 1. only good for one concentration 2. only truly accurate if constant temperature is applied throughout the entire process. So, what does all of this mean anyway? Bottom line it for me!! By changing the temperature at which we anneal steel and changing the length of time it is annealed, we can get various phases (microstructures) to form. Materials Science 03/06/16 4/4 Lecture 10 Coarse Pearlite - easy diffusion of carbon creates thick layers of iron carbide and alphaferrite Fine Pearlite - limited diffusion of carbon creates thin layers of iron carbide and alphaferrite Bainite 1. iron carbide “needles” in a ferrite matrix; fig 10.8 2. formed below the pearlite knee 3. pearlite vs. bainite is competitive 4. why do we want bainite? Spheroidite 1. formed when heating pearlite/bainite for 18+ hours near the eutectoid temp. 2. not shown on graph 3. sphere’s of iron carbide in an alpha-ferrite matrix (fig 10.10) 4. why do we want spheroidite? Martensite 1. formed from rapidly quenching to ambient temperatures 2. martensitic transformations - diffusionless transformations 3. wholesale changes in unit cell structure from bcc to bct with interstitial carbon 4. will vanish if heated but will stay indefinitely at room temperature why do we want martensite? Why do we care at all?