1 Indentifying Unknown #M20 via Infrared Spectroscopy, Mass
advertisement

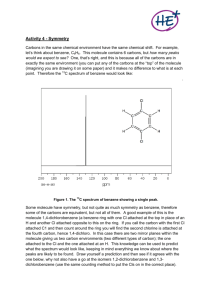
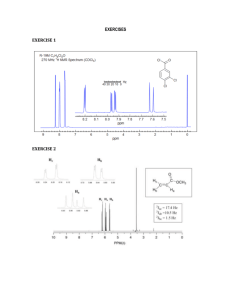
1 Indentifying Unknown #M20 via Infrared Spectroscopy, Mass Spectrometry and 13Carbon Nuclear Magnetic Resonance Spectroscopy Monica DiFiori November 8, 2013 Elementary Organic Chemistry 211 Prof. Rau 2 Abstract The objective of the experiment described below was to identify an unknown, #M20, via infrared spectroscopy, mass spectrometry and 13C NMR spectroscopy. The infrared spectrum suggested a ketone functional group was present. Combining that information with the identified molecular weight from the mass spectrum, the molecular formula, C6H10O was determined. Collectively analyzing the data presented in all three 13C NMR spectra, the symmetry and bonding environments of the carbon atoms within the compound lead to the final structure consisting of a six-carbon ring with a double-bonded oxygen. Introduction The purpose of the experiment was to use infrared spectroscopy, mass spectroscopy, and 13 C NMR spectroscopy to identify an unknown compound. Each technique suggests key information such as functional group, molecular weight, symmetry and bonding environments helpful for determining the molecular formula and structure of the unknown. Determining the functional group present in an unknown compound is important because that information is also used to determine the molecular weight and the architecture of the compound. Covalent bonds absorb infrared radiation differently, depending on the masses of incorporated atoms and the strength and polarity of the bonds involved (Rau, 2013). Thus, running infrared radiation on the unknown compound and measuring the absorbance at different wavelengths sheds light on the various bonds between atoms within the compound. Because bonds elicit different absorbance, the functional group can be determined. The spectrum produced is relative absorbance versus wavelength. This, in addition to mass spectrometry help determine the unknown compound’s molecular formula and structure. Mass spectrometry is used to determine the molecular weight of a molecule and elements of its structure. First the compound is flash vaporized via gas chromatography to separate impurities. The evaporated compound is bombarded with high-energy particles, forcing the once neutral molecules to gain a charge. Next, the now ions are accelerated via an external electric 3 field and sorted by mass and charge, using the relationships between force, mass, and velocity to classify the ions. Because the acceleration reached by an object is inversely related to it’s mass, a heavier ion will travel slower than lighter ion (Rau, 2013). The spectrum created shows the relative abundance of ions versus the mass/charge ratio (which is also equivalent to grams per mole). The molecular ion peak represents the molecular weight of the molecule. 13 C nuclear magnetic resonance spectroscopy (NMR) determines the specific arrangement of carbon atoms in a molecule. 13C NMR specifically involves interactions of electromagnetic radiation and magnetic fields with atomic nuclei of 13C isotopes. All NMRactive nuclei have an odd number of nuclear particles because the uneven number of particles means uneven pairing of spin. Because there’s one unmatched particle, the entire nucleus has a spin. A nucleus that has a spin also has a small magnetic field. When in the presence of another magnetic field, more 13C nuclei adopt to a lower energy spin state because the nucleus’s small magnetic field is opposite of the stronger magnetic field (Rau, 2013). Energy in the form of radiation is added to induce the change from lower energy spin states to higher energy spins states. Molecules with 13C atoms absorb radio wave energy varying with the bonding environment of each carbon (Rau, 2013). Thus, the NMR spectrum is a graph of the amount of radiation a nucleus needs to undergo the transition versus the wavelength of radiation. The spectrum indicates chemical shift values that determine each unique carbon’s bonding environment. Knowing the carbons’ bonding environment helps determine the specific arrangement of carbons within the molecule. In addition, knowing the number of different carbons indicates the level of symmetry in the compound. Combining all the information provided from all three techniques, the molecular formula and structure can be determined. Applying the information gathered from the IR spectrum to the 4 information gathered from the mass spectrometry, the molecular formula can be calculated. In addition to discovering the unknown molecule’s formula, the degrees of unsaturation can also be determined by analyzing the information presented in the mass spectrum. By comparing the determined formula to the fully saturated version of the formula, the degrees of unsaturation can de calculated and will shed light on the number of double and/or triple bonds and the possibility of a ringed structure. Once the molecular formula is determined based on the information presented in both the IR and mass spectra, the 13C NMR contributes by determining symmetry and carbon bonding environments. Three spectra are created from 13C NMR: carbon-proton decoupled (CPD), distortionless enhancement bi-polarization transfer (DEPT), and attached-proton test (APT). The CPD spectrum denotes each unique carbon bonding environment with single peaks. The number peaks demarcated in the spectrum compared to the number of carbons in the molecule reveals the level of symmetry within the molecule. The DEPT spectrum denotes all carbons bonded to one hydrogen and three hydrogen as positive peaks, all carbons bonded to two hydrogen as negative peaks and does not display single carbons (carbons with no bonds to hydrogens). The final spectrum, APT denotes carbons bonded to one or three hydrogen as positive peaks as in the DEPT and denotes carbons bonded to no hydrogens or two hydrogens as negative peaks. By comparing data between the three structures, the number of hydrogens bonded to each specific carbon can be determined. Pooling all the information gathered from all the spectra generated, the molecule’s molecular formula and structure is eventually identified. 5 Experimental A vial of unknown liquid, #M20, was obtained and was first run through IR spectroscopy. After cleaning the ATR sampler with acetone and a kim-wipe, one drop of the unknown liquid was placed on the ATR sampler. The program was run and it generated an IR spectrum. Next, the unknown compound was prepped for mass spectrometry. First, one drop of the unknown sample was placed in a 50 mL Erlenmeyer flask. Next, 5.0 mL of acetone was added to flask. After swirling the flask to ensure that the sample solution was evenly mixed, 1-1.5 mL was used to wash out the mass spectrometry vial. Rinsing out the vial in this manner ensures that the sample solution has no impurities. When thoroughly rinsed, the vile was filled to the third line with the sample solution, capped and labeled appropriately. After running the sample through mass spectrometry, a spectrum revealing the molecular weight was generated. The final part to the procedure was preparation of the unknown for 13C NMR. Before measuring and recording the exact mass of the unknown compound used for 13C NMR, an Erlenmeyer flask with an empty test tube inside was placed on the scale. Then, re-zeroing the scale, the unknown was added drop by drop until a sample with a mass of 2.5-3.5 g was reached. Next, 0.75 mL of CDCl3 was added to the test tube. Once the test tube was swirled, the sample solution was transferred to an NMR tube, which was capped and labeled appropriately. The sample was run and three spectra were produced. 6 Results Figure 1: Infrared Spectroscopy of Unknown Compound C:\Xcalibur\...\Ex3 C13 NMR\Ex350 DiFiori W23 A 9/24/2013 1:59:31 PM RT: 0.00 - 11.52 NL: 1.69E7 TIC F: MS Ex350 4.32 100 90 Relative Abundance 80 70 60 50 40 Figure 1:30The spectrum depicted above suggests a ketone functional group is present in the unknown molecule because the very intense peak (A) that appears at approximately 1704 wavenumbers represents the carbonyl bond. A 20 ketone functional group was determined rather than an ester functional group due to the peak appearing below 1730 wavenumbers. 10 3.34 3.76 0 0 2 6.88 4.46 5.29 4 8.01 6 9.25 10.27 11.28 8 10 98.01 99.04 Relative Abundance Figure 2: Mass Spectrometry of Unknown Compound Time (min) #275 RT: 4.31 AV: 1 NL: 2.95E6 Ex350 T: + c Full ms [ 60.00-300.00] 100 90 M+1 80.12 80 70 60 50 83.17 40 69.05 79.13 30 20 70.04 81.12 10 65.13 84.18 88.98 77.13 63.15 71.10 0 65 70 75 80 m/z 85 97.10 91.17 90 96.25 95 100 Figure 2: The spectrum produced from mass spectroscopy, pictured above, suggests the molecular weight of the unknown. The molecular ion peak (M+1), appearing on the far right, indicates a molecular weight of 98 g/mol. Calculations: To find molecular formula with determined weight of 98 g/mol (Figure 2): Mwt. of molecule – Mwt. of functional group = Mwt. of hydrocarbon structure (Mwt. of hydrocarbons ÷ amu of Carbon) = probable number of Carbons in molecule (probable number of Carbons)(amu of Carbon) = Mwt. of carbon within molecule mwt. of hydrocarbons – Mwt. of carbon within molecule = probable number of Hydrogens number of Carbons + number of Hydrogens = Hydrocarbon formula hydrocarbon formula + functional group formula = entire molecular formula 98 g/mol – 28 g/mol = 70 g/mol (70 g/mol ÷ 12 g/mol) ≈ 5 Carbons (5)(12 g/mol) = 60 g/mol Carbon 70 g/mol – 60 g/mol = 10 Hydrogens 5 Carbons + 10 Hydrogens = C5H10 deduced hydrocarbon molecular formula: C5H10 C5H10 + CO = C6H10O deduced molecular formula = C6H10O To find possible structures: Compare molecule to fully saturated molecule and subtract the number hydrogens in deduced molecule from the fully saturated molecule, then dividing by two to get degrees of unsaturation. 7 8 compare C6H10 to C6H14 14 - 10 = 4 4÷2=2 deduced degrees of unsaturation: 2° possible structures: 1) 2) 3) Figure 3: 13C NMR Spectroscopy CPD of Unknown Compound C D B E A Figure 3: The above spectrum indicates four unique carbon-bonding environments. The small peak (B), at approximately 77 ppm, is the solvent that is used in the sample preparation and therefor dismissed. 9 Figure 4: 13C NMR Spectroscopy DEPT of Unknown Compound C E D Figure 4: The DEPT spectrum shown above depicts three unique carbons-bonding environments occupied by carbons attached to two hydrogens represented by the three negative peaks (A, B, C). Also note that the solvent peak and the small peak that appeared around 210 ppm in the CPD are not present because single carbons are not shown in DEPT. Figure 5: 13C NMR Spectroscopy APT of Unknown Compound B A E D Figure 5: The spectrum depicted above suggests four uniquely bonded carbons when negating the solvent peak (B). Because the spectrum only indicates negative absorbance peaks, it suggests that all carbons present in the unknown are single carbons or carbons bonded to two hydrogens. C 10 Table 1: Carbon Signals within 13C NMR Spectra Peak Label Within Spectra A B C D E Chemical Shift Value (ppm) 210 77 41 26 24 Number of Carbon Atoms in Compound 1 0 2 2 1 Discussion In order to achieve the goal of determining the molecular formula and eventually the structure of an unknown compound, three different techniques are utilized. In order to discover the functional group within the unknown molecule, IR spectroscopy was used. Because all bonds absorb infrared light uniquely, the nature of the bonding within the unknown is revealed through measurements during radiation. Then, mass spectrometry is performed to further identify the molecular weight and formula. After gas chromatography, which flash vaporizes the molecule to ensuring purity, the evaporated compound is hit with it with high-energy particles and then put through an acceleration phase, which classifies the ions based on their mass to charge ratio. In addition to the produced IR and mass spectra, 13C NMR spectroscopy indicates the details of carbon bonding. The radiation of unevenly paired spins in NMR active nuclei, induces a transition from a lower energy spin state to a higher energy spin state. The spectra yielded illustrate chemical shift values and the number of differently bonded carbons, including their relationships with hydrogen. By collectively analyzing all spectra produced from the three techniques, the unknown can be identified. The first piece of the puzzle, the functional group was determined to be a ketone due to the carbonyl peak (A in Figure 1) at approximately 1704 wavenumbers. Combining that information with the information presented in the mass spectra, the molecular 11 formula was discovered. When looking at the mass spectra (Figure 2) the molecular ion peak (A in Figure 2), which was determined to be an even numbered value because the unknown was determined to have no nitrogen atoms, appears at approximately 98 g/mol. To calculate the molecular formula, the molecular weight of the functional group is subtracted from the total molecular weight. To help determine the structure, the degrees of unsaturation, which indicate double and/or triple bonds and ring formation, is determined by comparing the molecular formula, C6H10O, to the fully saturated version of the molecular formula, C6H14. The resulting two degrees of unsaturation indicates a double bond, due to the ketone functional group and an additional double bond or a ring formation. Additionally, to help identify the structure, the spectra yielded from 13C NMR is analyzed to determine the carbons in unique bonding environments, and symmetry within the molecule. The first spectrum, CPD (Figure 3) illustrates all different carbons within the molecule and suggests that the unknown molecule has four distinctive carbons out of a total of six carbons. As demonstrated in Table 1, the carbon signal labeled B is negated because it is a result of the solvent. Peak A, (Table 1) has a chemical shift value that suggests a carbonyl bond because electronegative bonds appear to the far left of the spectrum. This also confirms the functional group suggested by the IR spectrum. The peaks on the right side of the spectrum (C, D, E in Table 1) have chemical shift values that suggest a ring formation. Next, looking at the DEPT spectrum (Figure 4) the presence of the single carbon double bonded to an oxygen atom and thus the ketone functional group is confirmed again because there is no peak present above 200 ppm as in the CPD spectrum. Moreover, because all peaks (A, B, C in Figure 4) are negative, the three carbons indicated have two attached hydrogens. To confirm, the APT spectrum depicts only negative peaks (A, B, C, D, E,) as well. 12 The single carbons appear again at approximately 210 ppm (peak A in Figure 5) and at approximately 77 ppm (peak B in Figure 5). In order to determine the final correct structure, the proposed structures are tested against the information presented in the 13C NMR spectra. The second proposed structures fits the spectra, while the other two proposed structures do not. The ring of single bonded carbons contains four different carbon-bonding environments the first of which is represented as peak A throughout all 13C NMR spectra and as carbon A in Figure 6 (see below) and is the most electronegative carbon in the compound. The second carbon-bonding environment is represented as peak C, encompassing two similar carbons. These similar carbons are represented as C1 and C2 in Figure 6. Moving from more electronegative to less electronegative, the next carbonbonding environment represented in the 13C NMR spectra is peak D, shown as D1 and D2 in Figure 6 because two carbons occupy that same bonding environment. The last carbon in the compound is referred to as peak E in the 13C NMR spectra and carbon E in Figure 6. Figure 6: Detailed Carbon Labeling of Determined Final Structure C1 D1 E D2 A C2 Conclusion The goal of the experiment was to correctly identify various elements of an unknown compound using IR spectroscopy, mass spectrometry, and 13C NMR. First by analyzing the IR 13 spectrum, a ketone functional group was determined. The molecular ion peak found at 98 g/mol shown in the mass spectrum was used to determine the molecular weight, formula and ultimately the degree of unsaturation. Lastly, the spectra presented through the 13C NMR revealed a carbonyl peak at approximately 210 ppm and three other unique carbons with chemical shift values that suggests a ring structure. In combination with the APT, the DEPT spectrum indicated one single carbon and three carbons with two hydrogens attached to each. The only structure that satisfies all information presented from the respective spectra is a monosubstituted sixcarbon ring, with a double bonded oxygen, representing the ketone functional group. 14 References Rau, D. Elementary Organic Chemistry 1 Lab Manual. 2013