Unsaturated Hydrocarbons Solutions
advertisement
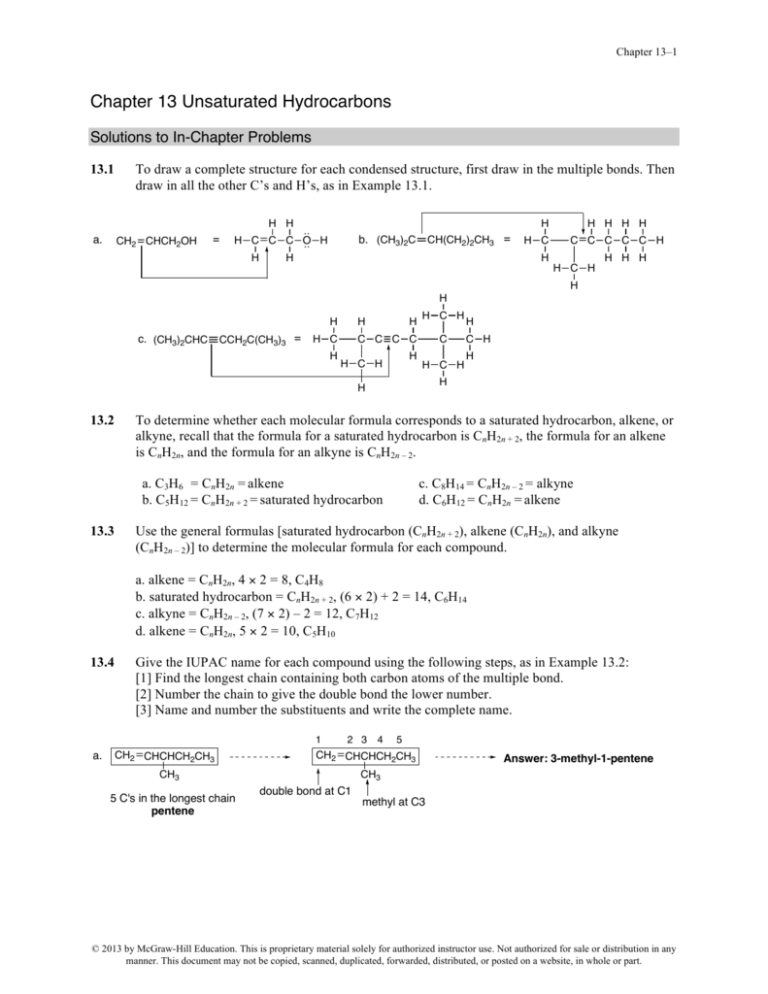
Chapter 13–1 Chapter 13 Unsaturated Hydrocarbons Solutions to In-Chapter Problems 13.1 To draw a complete structure for each condensed structure, first draw in the multiple bonds. Then draw in all the other C’s and H’s, as in Example 13.1. H H H a. CH2 CHCH2OH = H CH(CH2)2CH3 = b. (CH3)2C H C C C O H H C H H H H C C C C C H H H H H H H C H H H H c. (CH3)2CHC CCH2C(CH3)3 = H H H C C C C C H H H C H H C H C H C H H To determine whether each molecular formula corresponds to a saturated hydrocarbon, alkene, or alkyne, recall that the formula for a saturated hydrocarbon is CnH2n + 2, the formula for an alkene is CnH2n, and the formula for an alkyne is CnH2n – 2. a. C3H6 = CnH2n = alkene b. C5H12 = CnH2n + 2 = saturated hydrocarbon 13.3 C H H H 13.2 H c. C8H14 = CnH2n – 2 = alkyne d. C6H12 = CnH2n = alkene Use the general formulas [saturated hydrocarbon (CnH2n + 2), alkene (CnH2n), and alkyne (CnH2n – 2)] to determine the molecular formula for each compound. a. alkene = CnH2n, 4 × 2 = 8, C4H8 b. saturated hydrocarbon = CnH2n + 2, (6 × 2) + 2 = 14, C6H14 c. alkyne = CnH2n – 2, (7 × 2) – 2 = 12, C7H12 d. alkene = CnH2n, 5 × 2 = 10, C5H10 13.4 Give the IUPAC name for each compound using the following steps, as in Example 13.2: [1] Find the longest chain containing both carbon atoms of the multiple bond. [2] Number the chain to give the double bond the lower number. [3] Name and number the substituents and write the complete name. 1 a. CH2 CHCHCH2CH3 2 3 CH3 5 C's in the longest chain pentene 4 5 CH2 CHCHCH2CH3 Answer: 3-methyl-1-pentene CH3 double bond at C1 methyl at C3 © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–2 b. (CH3CH2)2C CHCH2CH2CH3 ethyl at C3 CH2CH3 CH3CH2C CHCH2CH2CH3 3 2 CH2CH3 CH3CH2C CHCH2CH2CH3 1 5 4 7 C's in the longest chain heptene double bond at C3 7 c. CH3CH2CH CHCH=CHCH3 4 3 2 2 5 CH2CH3 Answer: 3-ethylcyclopentene CH2CH3 4 5 C's in the ring cyclopentene ethyl at C3 Give the IUPAC name for each compound using the following steps, as in Example 13.2: [1] Find the longest chain containing both carbon atoms of the multiple bond. [2] Number the chain to give the multiple bond the lower number. [3] Name and number the substituents and write the complete name. CH3CH2CH2CH2CH2C CCH(CH3)2 CH3 CH3CH2CH2CH2CH2C CCHCH3 CH3 CH3CH2CH2CH2CH2C CCHCH3 5 9 C's in the longest chain nonyne CH2CH3 CH3CH2 C C CH2 C CH3 CH3 8 C's in the longest chain octyne 13.6 Answer: 2,4-heptadiene 3 d. b. 1 double bonds at C2 and C4 1 a. 5 6 CH3CH2CH CHCH=CHCH3 7 C's in the longest chain heptadiene 13.5 Answer: 3-ethyl-3-heptene 7 6 4 32 Answer: 2-methyl-3-nonyne 1 triple bond at C3 6 1 2 3 4 5 7 8 CH2CH3 CH3CH2 C C CH2 C CH3 Answer: 6,6-dimethyl-3-octyne CH3 triple bond at C3 2 methyl groups at C6 To draw the structure corresponding to each name, follow the steps in Example 13.3. • Identify the parent name to find the longest carbon chain or ring, and then use the suffix to determine the functional group; the suffix -ene = alkene and -yne = alkyne. • Number the carbon chain or ring and place the functional group at the indicated carbon. Add the substituents and enough hydrogens to give each carbon four bonds. 1 2 a. 4-methyl-1-hexene 6 carbon chain double bond at C1 3 4 5 6 C C C C C C CH3 CH2 CHCH2CHCH2CH3 CH3 methyl at C4 © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–3 1 2 b. 5-ethyl-2-methyl-2-heptene 3 4 5 6 7 CH3C CHCH2CHCH2CH3 C C C C C C C CH3 7 carbon chain double bond at C2 methyl at C2 1 c. 2,5-dimethyl-3-hexyne CH3 CH2CH3 ethyl at C5 2 3 4 5 6 CH3 C C C C C C 6 carbon chain triple bond at C3 CH2CH3 CH3 CH3CHC CH3 methyl at C2 CCHCH3 CH3 methyl at C5 CH2CH2CH3 1 CH2CH2CH3 4C C !d. 1-propylcyclobutene 3 4 carbon ring double bond at C1 C C propyl at C1 2 1 e. 1,3-cyclohexadiene 6 carbon ring 2 double bonds (C1 and C3) 3 5 4 10 f. 4-ethyl-1-decyne 2 6 9 8 7 6 5 4 2 1 CH3CH2CH2CH2CH2CH2CHCH2C CH2CH3 10 carbon chain triple bond at C1 13.7 3 C C C C C C C C C C CH CH2CH3 ethyl at C4 To draw the structures of the cis and trans isomers, follow the steps in Example 13.4. • Use the parent name to draw the carbon skeleton and place the double bond at the correct carbon. • Use the definitions of cis and trans to draw the isomers. When the two alkyl groups are on the same side of the double bond, the compound is called the cis isomer. When they are on opposite sides, it is called the trans isomer. H a. cis-2-octene 8 carbon chain CH3CH CHCH2CH2CH2CH2CH3 1 2 3 4 5 6 7 8 H C CH3 C CH2CH2CH2CH2CH3 cis isomer both alkyl groups on the same side H b. trans-3-heptene 7 carbon chain CH3CH2CH CHCH2CH2CH3 1 2 3 4 5 6 7 CH2CH2CH3 C CH3CH2 C H trans isomer alkyl groups on opposite sides © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–4 CH3 H c. trans-4-methyl-2-pentene C CH3CH CHCH(CH3)CH3 1 5 carbon chain 2 3 4 CHCH3 C CH3 5 H trans isomer alkyl groups on opposite sides 13.8 Whenever the two groups on each end of a C=C are different from each other, two isomers are possible. CH3 a. CH3CH2CH CHCH3 c. Each C has one H and one alkyl group. Cis and trans isomers are possible. CH3 C CHCH2CH2CH3 two CH3's cannot have cis or trans isomers b. CH2 CHCH2CH2CH3 two H's cannot have cis or trans isomers 13.9 When the two alkyl groups are on the same side of the double bond, the compound is called the cis isomer. When they are on opposite sides, it is called the trans isomer. cis H H H C C C C HOCH2(CH2)7CH2 CH2CH2CH3 H trans 13.10 a. Stereoisomers differ only in the three-dimensional arrangement of atoms. Constitutional isomers differ in the way the atoms are bonded to each other. CH3CH CHCH2CH3 and C bonded to one H, one CH3 CH2 CHCH2CH2CH3 C bonded to two H's different connectivity constitutional isomers b. CH3CH2 C C H CH3CH2 CH3 C C and H H C C CH3 H CH3 H CH3CH2 c. CH3CH2 and H C bonded to one CH2CH3 and one CH3 CH3 C C H H C bonded to one H and one CH2CH3 different connectivity constitutional isomers cis isomer trans isomer same connectivity different 3-D arrangement stereoisomers © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–5 13.11 Double bonds in naturally occurring fatty acids are cis. H H CH3CH2CH2CH2CH2 C C CH2 H H H H H H C C C C C C CH2 O CH2CH2CH2COH CH2 arachidonic acid 13.12 13.13 Fats are solids at room temperature because of their higher melting point. They are formed from fatty acids with few double bonds. Oils are liquids at room temperature because of their lower melting points. They are also formed from fatty acids, but have more double bonds. CH3(CH2)14COOH CH3(CH2)5CH=CH(CH2)7COOH palmitic acid no double bonds higher melting point 63 °C palmitoleic acid one double bond lower melting point 1 °C The functional groups in tamoxifen are labeled. ether amine OCH2CH2N(CH3)2 aromatic ring aromatic ring C C CH3CH2 aromatic ring alkene 13.14 The functional groups in RU 486 and levonorgestrel are labeled. amine aromatic ring hydroxyl hydroxyl alkyne (CH3)2N CH3 OH C alkene C alkyne CH3CH2 OH C CH CH3 ketone ketone O RU 486 O alkene alkene levonorgestrel © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–6 13.15 To draw the product of a hydrogenation reaction, use the following steps, as in Example 13.5: • Locate the C=C and mentally break one bond in the double bond. • Mentally break the H–H bond of the reagent. • Add one H atom to each C of the C=C, thereby forming two new C–H single bonds. a. CH2CH(CH3)2 CH3 b. C Pd H2 C CH3 Pd H CH3 CH3CH2CH2CH2CH2CH3 CH3CHCH2CH2CH(CH3)2 CH3 CH3 H2 c. 13.16 H2 CH3CH2CH CHCH2CH3 Pd To draw the product of each halogenation reaction, add a halogen to both carbons of the double bond. Cl Cl a. CH3CH2CH CH2 + Cl2 CH3CH2C C H H H Br CH3 CH3 b. + Br2 CH3 13.17 CH3 Br In hydrohalogenation reactions, the elements of H and Br (or H and Cl) must be added to the double bond. When the alkene is unsymmetrical, the H atom of HX bonds to the carbon that has more H’s to begin with. H H a. CH3CH CHCH3 + HBr CH3C CCH3 Br H This C does not have any H's. Add Br here. Br CH3 CH3 + b. HBr This C has more H's. Add H here. H H This C does not have any H's. Add Cl here. CH3 H c. (CH3)2C CHCH3 + HCl CH3 C Cl CCH3 H This C has more H's. Add H here. © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–7 d. + H Cl HCl H H 13.18 In hydration reactions, the elements of H and OH are added to the double bond. In unsymmetrical alkenes, the H atom bonds to the less substituted carbon. a. HO H H OH CH3CH CHCH3 CH3C CCH3 H2SO4 H H This C has one H so the OH bonds here. b. CH3CH2CH CH2 HO H H OH CH3CH2C C H H2SO4 H H This C has 2 H's so the H bonds here. CH3 c. CH3 13.19 OH CH3 H OH H2SO4 CH3 H Draw the products of each reaction. a. b. H2 CH3CH2CH2CH CH2 Pd Cl2 CH3CH2CH2CH CH2 H H CH3CH2CH2C C H H H H H CH3CH2CH2C C H Cl Cl c. Br2 CH3CH2CH2CH CH2 Br Br CH3CH2CH2C C H H H H H d. CH3CH2CH2CH CH2 H Br CH3CH2CH2C C H Br H e. CH3CH2CH2CH CH2 H Cl H H CH3CH2CH2C C H Cl H f. CH3CH2CH2CH CH2 H OH H2SO4 HO H CH3CH2CH2C C H H H © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–8 13.20 Draw the products of the hydrogenation reactions. H H a. H H H C C CH3CH2 C C CH2 H C C CH2 CH2CH2CH2CH2CH2CH2CH2COOH H2 Pd C C C C CH2CH2CH2 CH2 CH3CH2 H H C C CH3CH2 13.21 CH2CH2CH2CH2CH2CH2CH2COOH H H C C b. H H H H CH2 CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2COOH CH3CH2CH2CH2CH2CH2CH2 CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2COOH To draw the polymers, draw three or more alkene molecules and arrange the carbons of the double bonds next to each other. Break one bond of each double bond, and join the alkenes together with single bonds. With unsymmetrical alkenes, substituents are bonded to every other carbon. Use Example 13.7 as a guide. Join these 2 C's. CH3 a. CH2 Join these 2 C's. CH3 C CH2 CH3 C CH2CH3 CH2 H CH3 H CH3 H CH3 C CH2CH3 C C CH2CH3 C C H CH2 H CH2 H CH2 CH3 CH3 Join these 2 C's. CH3 b. CH2 C C CN CH2 C CN Cl C C C C C C C H CN H CN H CN Join these 2 C's. H CH2 H CH3 H CH3 H CH3 C CN H c. CH3 CH2 Join these 2 C's. CH3 Join these 2 C's. CH3 CH2 C C H CH2 Cl C H H H H H H C C C C C C H H H Cl Cl Cl Cl © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–9 13.22 Work backwards to determine what monomer is used to form the polymer. Break these bonds to form the monomer. H H H CH2C CH2C CH2C O O O COCH3 COCH3 COCH3 H formed from CH2 C O COCH3 poly(vinyl acetate) 13.23 Name each aromatic compound as in Example 13.8. Name the substituents on the benzene ring. With two groups, alphabetize the substituent names and use the prefix ortho, meta, or para to indicate their location. With three substituents, alphabetize the substituent names, and number to give the lowest set of numbers. OH CH2CH2CH3 OH on benzene ring = phenol propyl a. m-butylphenol c. propylbenzene CH3 CH2CH3 butyl CH2CH2CH2CH3 ethyl Br b. CH3 on benzene ring = toluene bromo d. I Cl 2-bromo-5-chlorotoluene p-ethyliodobenzene iodo 13.24 chloro Draw the structure corresponding to each name. NH2 a. pentylbenzene !!!c. m-bromoaniline CH2CH2CH2CH2CH3 Br Cl b. o-dichlorobenzene CH2CH3 Cl CH2CH3 !!!d. 4-chloro-1,2-diethylbenzene Cl 13.25 Commercially available sunscreens contain a benzene ring. Therefore, compound (a) might be found in a sunscreen since it contains two aromatic rings. Compound (b) does not contain any aromatic rings. 13.26 Phenols are antioxidants because the OH group on the benzene ring prevents unwanted oxidation reactions from occurring. Of the compounds listed, only curcumin (b) contains a phenol group (OH on a benzene ring), making it an antioxidant. © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–10 13.26 Draw the products of each substitution reaction. • Chlorination replaces one of the H’s on the benzene ring with Cl. • Nitration replaces one of the H’s on the benzene ring with NO2. • Sulfonation replaces one of the H’s on the benzene ring with SO3H. Cl Cl Cl Cl Cl2 a. SO3 c. FeCl3 Cl Cl Cl Cl Cl SO3H Cl Cl NO2 HNO3 b. H2SO4 H2SO4 Cl 13.27 Cl Draw the products of the substitution reaction. The Cl can replace any of the H’s on the benzene ring, giving three different products. CH3 Cl2 CH3 CH3 CH3 Cl FeCl3 Cl Cl o-chlorotoluene m-chlorotoluene p-chlorotoluene Solutions to End-of-Chapter Problems 13.29 a. molecular formula: C10H12O b. aromatic ring, alkene, ether c. trans d. Tetrahedral C's are indicated. All other C's are trigonal planar. aromatic ring trans alkene ether O CH3 tetrahedral CH3 anethole tetrahedral 13.30 a. molecular formula: C10H12O2 b. aromatic ring, ester c. trans d. Tetrahedral C is indicated. All other C's are trigonal planar. trans alkene O aromatic ring O CH3 tetrahedral methyl cinnamate © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–11 13.31 Use the general formulas [saturated hydrocarbon (CnH2n + 2), alkene (CnH2n), and alkyne (CnH2n – 2)] to determine the molecular formula for each compound with 10 C’s. a. (10 × 2) + 2 = 22: molecular formula C10H22 b. 10 × 2 = 20: molecular formula C10H20 13.32 Draw the structure of the hydrocarbon fitting the required description. a. 13.33 c. (10 × 2) – 2 = 18: molecular formula C10H18 HC CCH2CH2CH2CH3 b. CH2 CHCH2CH2CH CH2 c. Draw three alkynes with molecular formula C5H8. CH3 HC CCH2CH2CH3 CH3C CCH2CH3 HC C C H CH3 13.34 13.35 Draw the five constitutional isomers of C5H10 that contain a double bond. CH2 CHCH2CH2CH3 CH3CH CHCH2CH3 CH3 CH3C CHCH3 CH3 CH2 CHCHCH3 CH3 CH2 CCH2CH3 Label each carbon as tetrahedral, trigonal planar, or linear by counting groups. tetrahedral trigonal planar tetrahedral a. c. trigonal planar tetrahedral trigonal planar linear b. C CH all trigonal planar 13.36 Predict the indicated bond angles in falcarinol. a b OH c H C C C C C C C CH2 H H H d a. trigonal planar: 120° C C (CH2)6CH3 H H e b. tetrahedral: 109.5° c. inear: 180° © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–12 13.37 Give the IUPAC name for each compound. a. b. 1 2 1 2 3 3 4 4 5 6 6 C chain hexene 2-ethyl 4 C chain butene 13.38 2-hexyne 2-ethyl-1-butene Give the IUPAC name for each compound. 1 a. HC C CH2CH2CH2CH2CH3 7 C chain 13.39 a. CH2CH3 1- heptyne 5 carbon ring; ethyl at C2 2-ethylcyclopentene Give the IUPAC name for each compound using the following steps, as in Example 13.2: [1] Find the longest chain containing both carbon atoms of the multiple bond. [2] Number the chain to give the multiple bond the lower number. [3] Name and number the substituents and write the complete name. CH2 CHCH2CH2C(CH3)3 CH3 1 CH2 CHCH2CH2CCH3 2 3 4 CH3 CH3CH2C CH3 double bond at C1 2 methyls at C5 CHCHCH2CHCH3 CH2 C CH2CH3 CH2CH2CH2CH2CH3 7 C's in the longest chain heptene 2 methyls at C5 and C7 ethyl at C3 CH3CH2 CH3 8 C's in the longest chain octene c. Answer: 5,5-dimethyl-1-hexene CH3 CHCHCH2CHCH3 CH3CH2 CH3 CH3 6 C's in the longest chain hexene (CH3CH2)2C 5 CH2 CHCH2CH2CCH3 CH3 b. 2 b. CH3CH2C 1 2 CH3 CH3 CHCHCH2CHCH3 3 4 5 6 7 8 Answer: 3-ethyl-5,7-dimethyl-3-octene double bond at C3 1 2 ethyl at C2 CH2 C CH2CH3 Answer: 2-ethyl-1-heptene CH2CH2CH2CH2CH3 3 4 5 6 7 double bond at C1 © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–13 d. CH3C CCH2C(CH3)3 5 1 CH3 CH3C CCH2CCH3 2 3 CH3 Answer: 5,5-dimethyl-2-hexyne CH3C CCH2CCH3 CH3 6 C's in the longest chain hexyne triple bond at C2 CH3 6 2 methyls at C5 2 methyls at C5 and C6 5 CH3 CH3 1 e. CH3C C CH2 CH C CH2CH3 2 3 6 CH3 CH3 4 8 CH3C C CH2 CH C CH2CH3 CH2CH3 CH2CH3 Answer: 6-ethyl-5,6-dimethyl-2-octyne triple bond at C2 8 C's in the longest chain octyne ethyl at C6 3 CH3 f. 5 4 6 CH2 CHCH2 C CH=CH2 CH3 1 CH3 6 C's in the longest chain hexadiene 13.40 CH3 CH2 CHCH2 C CH=CH2 Answer: 3,3-dimethyl-1,5-hexadiene 2 methyls at C3 double bonds at C1 and C5 Give the IUPAC name for each compound using the following steps, as in Example 13.2: [1] Find the longest chain containing both carbon atoms of the multiple bond. [2] Number the chain to give the multiple bond the lower number. [3] Name and number the substituents and write the complete name. a. CH2 CHCH2CHCH2CH3 CH3 2 1 6 C's in the longest chain hexene b. (CH3)2C CHCH2CHCH2CH3 3 double bond at C1 double bond at C2 1 CH3 CH2CH2CH3 8 C's in the longest chain octene 6 Answer: 4-methyl-1-hexene CH3 CH2CH2CH3 CH3 C CHCH2CHCH2CH3 4 5 CH2 CHCH2CHCH2CH3 CH2 CHCH2CHCH2CH3 CH3 2 3 methyl at C4 ethyl at C5 5 CH3 C CHCH2CHCH2CH3 CH3 Answer: 5-ethyl-2-methyl-2-octene CH2CH2CH3 6 8 methyl at C2 © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–14 c. CH3C CCHCH3 triple bond at C2 methyl at C4 CH2CH2CH3 1 CH3C CCHCH3 2 4 Answer: 4-methyl-2-heptyne CH3C CCHCH3 CH2CH2CH3 CH2CH2CH3 7 5 7 C's in the longest chain heptyne d. (CH3)3CC CC(CH3)3 triple bond at C3 CH3 CH3 CH3 CH3CC CCCH3 CH3 6 5 6 C's in the longest chain hexyne CH3 3 CH3 2 4 methyls at C2 and C5 CHCH3 double bond at C2 3 7 CH3CH2CH2CH2C CHCH3 butyl at C3 f. (CH3)2C CHCH2CH2CH2CH C(CH3)2 double bonds at C2 and C7 1 CHCH2CH2CH2CH CCH3 CH3 9 C's in the longest chain nonene 13.41 2 CH3C 3 7 89 CHCH2CH2CH2CH CCH3 CH3 CH3 Answer: 2,8-dimethyl-2,7-nonadiene methyls at C2 and C8 Give the IUPAC name for each compound using the steps in Answer 13.39 and Example 13.2. double bond at C1 2 a. 1 Answer: 3-butyl-2-heptene CH2CH2CH2CH3 7 C's in the longest chain heptene CH3 1 CH3CH2CH2CH2C CHCH3 CH2CH2CH2CH3 CH3C Answer: 2,2,5,5-tetramethyl-3-hexyne CH3CC CCCH3 CH3 e. (CH3CH2CH2CH2)2C CH3 3 4 CH3 4-methylcyclohexene b. 2 1 methyl at C4 6 carbon ring cyclohexene 3 CH2CH3 CH2CH3 2 ethyl groups at C3 3,3-diethylcyclobutene 4 4 carbon ring cyclobutene © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–15 13.42 Give the IUPAC name for each compound using the steps in Answer 13.39 and Example 13.2. double bonds at C1 and C4 double bond at C1 a. 2 1 2 3 4 CH2CH2CH3 3 4 b. 1 4-propylcylcopentene 1,4-cycloheptadiene 5 7 carbon ring cycloheptene propyl at C4 5 carbon ring cyclopentene 13.43 To draw the structure corresponding to each name, follow the steps in Example 13.3. • Identify the parent name to find the longest carbon chain or ring, and then use the suffix to determine the functional group; the suffix -ene = alkene and -yne = alkyne. • Number the carbon chain or ring and place the functional group at the indicated carbon. Add the substituents and enough hydrogens to give each carbon four bonds. 1 2 a. 3-methyl-1-octene 8 carbon chain double bond at C1 4 5 6 7 8 CH2 CHCHCH2CH2CH2CH2CH3 CH3 CH3 methyl at C3 C C b. 1-ethylcyclobutene 4 carbon ring double bond at C1 CH2CH3 3 4 CH2CH3 ethyl at C1 C C 1 2 c. 2-methyl-3-hexyne 6 carbon chain triple bond at C3 3 C C C C C C C C 5 H 6 C C C C C C CH3 C C CCH2CH3 CH3 CH3 methyl at C2 1 2 CH2CH3 4 5 6 C C C C C C C d. 3,5-diethyl-2-methyl-3-heptene 3 CH3 7 carbon chain double bond at C3 1 2 3 4 5 6 8 carbon chain double bond at C2 3 4 5 CH2CH3 7 C C C C C C C 1 2 CH3 2 ethyl groups at C3 and C5 CH2 C C CHCH2CH2CH3 H H 7 carbon chain double bonds at C1 and C3 f. cis-7-methyl-2-octene CH2CH3 H H CH3 C C C CCH2CH3 CH2CH3 methyl at C2 !e. 1,3-heptadiene H 7 6 7 8 C C C C C C C C CH3 CH3 C C H CH2CH2CH2CHCH3 H CH3 cis methyl at C7 © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–16 13.44 To draw the structure corresponding to each name, follow the steps in Example 13.3. • Identify the parent name to find the longest carbon chain or ring, and then use the suffix to determine the functional group; the suffix -ene = alkene and -yne = alkyne. • Number the carbon chain or ring and place the functional group at the indicated carbon. Add the substituents and enough hydrogens to give each carbon four bonds. 1 CH3 1 CH3 a. 1,2-dimethylcyclopentene methyls at C1 and C2 b. 6-ethyl-2-octyne 8 carbon chain triple bond at C2 1 2 3 4 5 6 7 8 C C C C C C C C ethyl at C6 1 c. 3,3-dimethyl-1,4-pentadiene 5 carbon chain double bonds at C1 and C4 2 CH3 C CCH2CH2CHCH2CH3 CH2CH3 CH2CH3 CH3 CH3 4 5 C C C C C CH2 CH C CH CH2 CH3 CH3 2 methyls at C3 5 6 C C C C C C 1 d. trans-5-methyl-2-hexene 6 carbon chain double bond at C2 e. 5,6-dimethyl-2-heptyne 7 carbon chain triple bond at C2 2 CH 3 2 CH 3 5 carbon ring double bond at C1 2 3 4 methyl at C5 1 2 3 4 5 C C C C C CH3 H CH3 C C H CH2CHCH3 CH3 trans 6 7 C C CH3 C C CH2 CH3 CH3 CH CHCH3 CH3 CH3 methyls at C5 and C6 1 f. 3,4,5,6-tetramethyl-1-decyne 10 carbon chain triple bond at C1 13.45 3 4 5 6 C C C 2 C C C C C C C 7 8 9 10 CH C CH CH3 CH3 CH3 CH3 CH3 CH CH CH(CH2)3CH3 CH3 CH3 CH3 methyls at C3, C4, C5 and C6 Correct each of the incorrect IUPAC names. a. The name 5-methyl-4-hexene places the double bond at C4 instead of C2. Assign the lower number to the alkene: 2methyl-2-hexene. b. The name 1-methylbutene makes the last carbon in the chain a substituent. In addition, the location of the double bond is not specified. There are five carbons in the chain (not a methyl substituent): 2-pentene. c. The name 2,3-dimethylcyclohexene starts numbering substituents at C2 instead of C1. Number to put the C=C between C1 and C2, and then give the first substituent the lower number: 1,6-dimethylcyclohexene. CH3 CH3C CHCH2CH2CH3 2-methyl-2-hexene CH3 CH CHCH2CH3 2-pentene CH3 1 CH3 6 2 3 4 5 1,6-dimethylcyclohexene © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–17 1 d. The name 3-butyl-1-butyne does not name the longest chain. Name the seven carbon chain: 3-methyl-1-heptyne. 13.46 3 2H HC CCCH3 CH2CH2CH2CH3 3-methyl-1-heptyne Correct each of the incorrect IUPAC names. CH3 a. The alkene has two methyl groups off of C2. Therefore, it cannot be cis. b. The name 2-methyl-2,4-pentadiene places the double bonds at C2 and C4 instead of C1 and C3. The methyl group should be at C4. c. The name 2,4-dimethylcyclohexene starts numbering substituents at C2 instead of C1. Number to put the C=C between C1 and C2, and then give the first substituent the lower number: 1,5-dimethylcyclohexene. CH3C CHCH2CH2CH3 2-methyl-2-hexene CH3 C 4-methyl-1,3-pentadiene CH3 1 6 13.47 2 3 4 5 CH3 1,5-dimethylcyclohexene 1 d. The name 1,1-dimethyl-2-cyclohexene does not number the double bond between C1 and C2. The methyl groups should be on C3. CHCH CH2 CH3 6 2 5 3 CH3 4 CH3 3,3-dimethylcyclohexene When the two alkyl groups are on the same side of the double bond, the compound is called the cis isomer. When they are on opposite sides it is called the trans isomer. trans cis H CH3CH2CH2CH2CH2 C C H C H H H C C C CH2 H C H C H OH CHCHCH2CH2CH2COOH S CH2 CHCONHCH2COOH NHCOCH2CH2CHCOOH NH2 © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–18 13.48 Draw the structures using the proper cis or trans arrangement around the carbon–carbon double bond. cis a. CH3(CH2)8CH C C H (CH2)12CH3 H 13.49 b. trans C C H C H H O Give the IUPAC name for the alkene. Use the definition in Answer 13.47 to determine if it is the cis or trans isomer. 5 1 4 2 cis-4-methyl-2-pentene 3 cis 4-methyl 13.50 Give the IUPAC name for the alkene. Use the definition in Answer 13.47 to determine if it is the cis or trans isomer. CH3 4 1 H3C 2 3 2-methyl 13.51 6 7 CH3 5 trans trans-2-methyl-3-heptene Draw the cis and trans isomers for each compound, as in Example 13.4. a. H H C C CH2CH2CH2CH2CH2CH3 CH3 cis-2-nonene H CH2CH2CH2CH2CH2CH3 C C CH3 H H H b. CH3CH C C CH2CH2CH3 CH3 cis-2-methyl-3-heptene CH2CH2CH3 H C C CH3CH H CH3 trans-2-nonene trans-2-methyl-3-heptene © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–19 13.52 Draw the cis and trans isomers for each compound, as in Example 13.4. a. C C CH3 CH3 CH2CH3 cis-4,4-dimethyl-2-hexene CH3 CH2CH3 C C C CH3 CH3 H CH2CH2CH3 H C C CH3CH2 C CH3 cis-3-heptene H C C b. CH2CH2CH3 CH3CH2 H H H H H trans-3-heptene trans-4,4-dimethyl-2-hexene 13.53 Constitutional isomers have the same molecular formula, but have the atoms bonded to different atoms. Stereoisomers have atoms bonded to the same atoms but in a different three-dimensional arrangement. 13.54 Draw the possible stereoisomers for 2,4-hexadiene. H H C C C C CH3 13.55 CH3 H CH3 C C C C H H H H CH3 CH3 H C C C C H H H CH3 H Determine if the molecules are constitutional isomers, stereoisomers, or identical. a. and same molecular formula same connectivity identical b. and same molecular formula different connectivity constitutional isomers © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–20 13.56 Determine if the molecules are constitutional isomers, stereoisomers or identical. CH3 a. CH3 same molecular formula same connectivity identical a. CH3 H2 CH3CH2CH2CH2CH2CH3 c. (CH3)2CHCH2CH2CH2CH3 d. Pd H2 b. (CH3)2C a. CH3 Draw the products of each reaction by adding H2 to the double bond. CH2 CHCH2CH2CH2CH3 13.58 H C 3 and same molecular formula same connectivity different arrangement in space (cis and trans isomers) stereoisomers b. H3C and 13.57 CH3 CH3 CH3 CHCH2CH2CH3 Pd CH3 H2 Pd H2 CH2 H Pd CH3 Draw the products of each reaction by adding Br2 to the double bond. CH3 Br2 CH2 CHCH2CH2CH2CH3 CH2CHCH2CH2CH2CH3 Br2 c. CH3 Br Br Br Br Br2 b. (CH3)2C CHCH2CH2CH3 (CH3)2C CHCH2CH2CH3 d. Br2 CH2 Br CH2Br Br Br 13.59 Draw the products of each reaction by adding HCl to the double bond. Cl HCl a. b. (CH3)2C 13.60 C(CH3)2 c. Cl H HCl (CH3)2C d. C(CH3)2 HCl CH2 CHCH2CH(CH3)2 CH2 HCl CH3 Cl Draw the products of each reaction by adding H2O to the double bond. OH a. Cl CH3CHCH2CH(CH3)2 + H2O H2SO4 b. (CH3)2C C(CH3)2 + H2O c. CH2 CHCH2CH(CH3)2 + H2O H2SO4 OH H (CH3)2C C(CH3)2 d. CH2 + H2O H2SO4 H2SO4 OH CH3CHCH2CH(CH3)2 CH3 OH © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–21 13.61 Draw the products of each reaction by adding the specified reagent to the double bond. H CH2CH3 a. H2 CH2CH3 Pd H CH2CH3 Cl CH2CH3 HCl d. H H H Cl CH2CH3 Cl2 b. CH2CH3 CH2CH3 e. Br CH2CH3 HBr H H Cl H CH2CH3 c. Br CH2CH3 Br2 CH2CH3 f. H H2SO4 Br H 13.62 OH CH2CH3 H2O H Draw the products of each reaction by adding the specified reagent to the double bond. a. CH3CH CHCH3 + HCl CH3CHCH2CH3 d. CH3CH CHCH2CH2CH3 + Br2 CH3CHCHCH2CH2CH3 Br Br Cl b. + H2 Pd (CH3)2CCH2CH2CH3 e. (CH3)2C CHCH2CH3 + HBr Br CH3 c. Cl2 CH3 13.63 a. Cl Cl CH3 CH3 f. CH3 CH3 + H2O H2SO4 CH3 CH3 CH3 OH CH3 Work backwards to determine what alkene is needed as a starting material to prepare each of the alkyl halides or dihalides. CH2 CH2 HBr CH3CH2Br c. CH3 Cl2 Cl CH3 Cl HCl b. Cl d. CH2 CHCH2CH(CH3)2 Br2 BrCH2CHCH2CH(CH3)2 Br 13.64 Markovnikov’s rule must be followed when determining the starting materials. 2-Bromobutane can be formed as the only product of the addition of HBr to 1-butene and 2-butene. 2Bromopentane can be formed as the only product of the addition of HBr to 1-pentene. CH2 CHCH2CH3 + HBr CH3CHCH2CH3 CH3CH CHCH3 1-butene CH2 CHCH2CH2CH3 + HBr 2-bromobutane + HBr CH3CHCH2CH3 Br Br 2-butene 2-bromobutane CH3CHCH2CH2CH3 Br 1-pentene 2-bromopentane © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–22 13.65 Work backwards to determine what reagent is needed to convert 2-methylpropene to each product. a. (CH3)2C=CH2 b. (CH3)2C=CH2 c. 13.66 HCl H2 Pd H2O (CH3)2C=CH2 (CH3)3CCl d. (CH3)2C=CH2 (CH3)3CH e. (CH3)2C=CH2 (CH3)3CBr Br2 (CH3)2CCH2Br Br Cl2 f. (CH3)2C=CH2 (CH3)3COH H2SO4 HBr (CH3)2CCH2Cl Cl a. The addition of Br2 could be used to tell the difference between cyclohexane and cyclohexene. Br2 is red in color. There is no reaction when Br2 is added to cyclohexane, so the solution would remain red. The bromines will add across the double bond, though, when Br2 is added to cyclohexene, thus yielding colorless 1,2-dibromocyclohexane. b. The addition of Br2 could also be used to distinguish between cyclohexene and benzene. When Br2 is added to cyclohexene, 1,2-dibromocyclohexane is formed and the red color of the Br2 disappears. When Br2 is added to a solution containing benzene, the red color will remain because the benzene will not undergo a subsitution reaction, except in the presence of FeBr3. 13.67 To draw the polymer, draw three or more alkene molecules and arrange the carbons of the double bonds next to each other. Break one bond of each double bond, and join the alkenes together with single bonds. With unsymmetrical alkenes, substituents are bonded to every other carbon. Use Example 13.7 as a guide. Join these 2 C's. H CH2 H C CH2 CH2 C COOH COOH CH2C CH2C CH2C H H H Draw the polymer using the steps in Example 13.7. Join these 2 C's. CH3 CH2 Join these 2 C's. CH3 C CH2 COOCH3 13.69 COOH COOH COOH H C COOH 13.68 Join these 2 C's. COOCH3 COOCH3 COOCH3 CH3 C CH2 COOCH3 CH2C C CH2C CH3 COOCH3 CH2C CH3 CH3 Draw the polymers using the steps in Example 13.7. Join these 2 C's. CH2CH3 a. CH2 C CH2CH3 CH2 CH2CH3 C H CH2 C H Join these 2 C's. b. CH 2 Join these 2 C's. Cl C C CH3 CH2 CH2 CH2 H H H Join these 2 C's. Cl CH2 CN CH3 CH2C CH2C CH2C H Cl CH2 CN CH3 C CN Cl Cl Cl CH2C CH2C CH2C CN CN CN © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–23 Join these 2 C's. c. Cl CH2 Cl C CH2 CH2 C Cl Cl Cl CH2C CH2C CH2C Cl Cl Cl Cl Draw the polymers using the steps in Example 13.7. Join these 2 C's. a. CH2 Join these 2 C's. OCH3 OCH3 CH2 C CH2 CH2 CH2 Join these 2 C's. c. CH2 C CO2CH3 CH2 C H Cl Cl Cl CH2C CH2C CH2C CO2CH3 CO2CH3 CO2CH3 Join these 2 C's. H H H CH2C CH2C CH2C H C CH2 C NHCOCH3 NHCOCH3 CH2C H CO2CH3 H H CH2C H Cl C CO2CH3 OCH3 Join these 2 C's. Cl C OCH3 CH2C H Join these 2 C's. b. CH2 C H Cl OCH3 OCH3 C H 13.71 Cl Cl C Cl 13.70 Join these 2 C's. NHCOCH3 NHCOCH3 NHCOCH3 NHCOCH3 Work backwards to determine what monomer was used to form the polymer. Each one of these units is from the monomer: 13.72 Br Br Br CH2 C CH2 C CH2 C Cl Cl Cl Cl CH2 C Br Work backwards to determine what monomer was used to form the polymer. Each one of these units is from the monomer: CH3 CH3 CH2 C CH2 C C O CH2 C C O OCH2CH3 13.73 CH3 OCH2CH3 CH2 C CH3 C O C O OCH2CH3 OCH2CH3 Draw two resonance structures by moving the double bonds. Cl Cl © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–24 13.74 The structures A and B are the same compound even though A has the two Cl atoms on the same double bond and B has the two Cl atoms on different double bonds, because the two structures are resonance structures. They differ in the placement of the electrons, but the placement of the atoms is the same. 13.75 Name each aromatic compound as in Example 13.8. Name the substituents on the benzene ring. With two groups, alphabetize the substituent names and use the prefix ortho, meta, or para to indicate their location. With three substituents, alphabetize the substituent names, and number to give the lowest set of numbers. b. a. bromo fluoro chloro ethyl p-chloroethylbenzene 13.76 o-bromofluorobenzene Name each aromatic compound as in Example 13.8. Name the substituents on the benzene ring. With two groups, alphabetize the substituent names and use the prefix ortho, meta, or para to indicate their location. With three substituents, alphabetize the substituent names, and number to give the lowest set of numbers. a. OH propyl b. H2N bromo aniline phenol m-propylphenol 13.77 Br p-bromoaniline Name each aromatic compound as in Example 13.8 and Answer 13.75. Cl nitro NO2 a. butyl m-chloronitrobenzene c. o-butylethylbenzene ethyl chloro Cl b. H2N NO2 nitro p-nitroaniline OH OH on benzene ring = phenol Cl 2,5-dichloro d. 2,5-dichlorophenol NH2 on benzene ring = aniline © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–25 13.78 Name each aromatic compound as in Example 13.8. Name the substituents on the benzene ring. With two groups, alphabetize the substituent names and use the prefix ortho, meta, or para to indicate their location. With three substituents, alphabetize the substituent names, and number to give the lowest set of numbers. a. CH3(CH2)3 (CH2)3CH3 H2N c. NH2 on benzene ring = aniline Br ethyl CH2CH3 b. Br bromo CH3 o-bromoethylbenzene bromo ethyl m-ethylaniline p-dibutylbenzene 2 butyl groups CH2CH3 I iodo d. 2-bromo-3-iodotoluene CH3 on benzene ring = toluene 13.79 Draw and name the three isomers with Cl and NH2 as substituents. Recall that a benzene ring with an NH2 group is named aniline. NH2 NH2 NH2 Cl Cl o-chloroaniline 13.80 m-chloroaniline Cl p-chloroaniline Draw the structure of 2,4,6-trinitrotoluene (TNT). CH3 O2N NO2 NO2 13.81 Work backwards to draw the structure from the IUPAC name. NO2 nitro CH3 a. p-nitropropylbenzene CH2CH2CH2CH3 chloro Cl butyl OH on benzene ring = phenol iodo 2-bromo-4-chlorotoluene 4 m-dibutylbenzene b. CH3 on benzene ring = toluene bromo 2 Br 3 butyl CH2CH2CH2CH3 I d. propyl CH2CH2CH3 OH 1 NH2 1 e. I 6 5 NH2 on benzene ring = aniline 2 Cl 2-chloro-6-iodoaniline 3 4 iodo chloro o-iodophenol c. © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–26 13.82 Work backwards to draw the structure from the IUPAC name. NO2 m-ethylnitrobenzene a. CH2CH3 1,3,5-trinitrobenzene d. O 2N ethyl fluoro F F NO2 OH on benzene ring = phenol OH Br o-difluorobenzene b. nitro NO2 nitro e. bromo Br CH3 CH3 on benzene ring = toluene p-bromotoluene c. bromo Br 13.83 2,4-dibromophenol Draw the products of each reaction. SO3H Cl a. CH3 Cl2 CH3 FeCl3 CH3 c. CH3 CH3 CH3 SO3 H2SO4 CH3 CH3 NO2 b. CH3 13.84 HNO3 CH3 H2SO4 CH3 CH3 Determine the reagents for the reactions in the sequence. A B Cl Cl2 HNO3 H2SO4 FeCl3 Cl O 2N 13.85 SO3H Cl D O2N C SO3 H2SO4 Cl H2 Pd H2N SO3H Draw the three products formed in the reaction of bromobenzene. Br HNO3 H2SO4 Br Br NO2 Br NO2 NO2 © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–27 13.86 Draw the structures that result from the substitution of a chloro group onto the benzene ring. Br Br FeCl3 + Cl2 Br Cl Br Br + Cl2 Br Br FeCl3 + Br Cl Br Br Cl A C Br Br + Cl2 Br Br Cl FeCl3 + Br + Br Br B Cl Br Cl 13.87 Vitamin E is an antioxidant because of the phenol, which has an OH bonded to the benzene ring. 13.88 BHA is an ingredient in some breakfast cereals and other packaged foods because it is a synthetic antioxidant and can prevent oxidation and spoilage. 13.89 Methoxychlor is more water soluble than DDT. The OCH3 groups can hydrogen bond to water. This increase in water solubility makes methoxychlor more biodegradable. 13.90 2,4-D is soluble in water because it contains an –OCH2COOH group. This group can hydrogen bond to water through the oxygen bound to the benzene ring and the two oxygens on the carboxy group (COOH). DDT has no groups that are able to hydrogen bond to water, so DDT is insoluble in water. 13.91 H H H C C a. CH3CH2CH2CH2CH2 H C C CH2 CH2CH2CH2CH2CH2CH2CH2COOH H H H2, Pd C C CH2CH2CH2CH2CH2CH2CH2COOH CH3CH2CH2CH2CH2CH2CH2CH2 H H C C CH3CH2CH2CH2CH2 b. + Partial hydrogenation adds hydrogen to one of the double bonds. CH2 CH2CH2CH2CH2CH2CH2CH2CH2CH2COOH Complete hydrogenation adds hydrogen to both of the double bonds, forming: CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2COOH © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–28 c. CH2CH2CH2CH2CH2CH2CH2COOH H one possibility: C C H CH3CH2CH2CH2CH2CH2CH2CH2 trans 13.92 Draw the structures and determine which will have the higher melting point. a. CH3(CH2)2CH2 b. CH3(CH2)2CH2 H H C C H C C H c. 13.93 (CH2)7COOH H H H C C H C C H H C C H H C C (CH2)7COOH The all-trans isomer will have the higher melting point because it has a more linear shape than the isomer with the cis double bond. As a result, the all-trans isomer packs more closely together in the solid, and thus requires more energy to separate upon melting. Recall from Section 13.12 that many phenols are antioxidants. a. b. CH2CH2CH2OH an alcohol not an antioxidant c. HO OCH3 an ether not an antioxidant OCH3 This compound could be an antioxidant because it has an OH group bonded to the aromatic ring. 13.94 All commercial sunscreens contain a benzene ring. The structure in part a contains two benzene rings and therefore might be an ingredient in a commercial sunscreen. The structure in part b contains two cyclohexane rings and therefore would not be an ingredient in a commercial sunscreen. 13.95 When benzene is oxidized to phenol, it is converted to a more water-soluble compound that can then be excreted in the urine. 13.96 A PAH is a polycyclic aromatic hydrocarbon, a compound that contains two or more benzene rings that share carbon–carbon bonds. The structure of anthracene, a PAH mentioned in Section 13.10D, is shown below. 13.97 H H a. CH3(CH2)5 (CH2)7COOH cis palmitoleic acid 13.98 (CH2)7COOH H b. C C CH3(CH2)5 H trans stereoisomer H H c. C C C C CH3(CH2)4 (CH2)8COOH constitutional isomer one possibility Polyethylene is a long chain hydrocarbon. Water and carbon dioxide are formed when polyethylene undergoes combustion. © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–29 13.99 All the carbons in benzene are trigonal planar with 120° bond angles, resulting in a flat ring. In cyclohexane, all the carbons are tetrahedral, so the ring is puckered. 13.100 p-Dichlorobenzene is a nonpolar molecule but o-dichlorobenzene is a polar molecule because the p-dichlorobenzene molecule is symmetrical and therefore does not have a net dipole moment. oDichlorobenzene, on the other hand, has a net dipole. Cl Cl Cl Cl nonpolar the dipoles cancel polar 13.101 a. CH2=CH(CH2)4CH3 7 C chain 1-heptene b. CH2=CH(CH2)4CH3 c. CH2=CH(CH2)4CH3 d. polymerization: H2 CH3(CH2)5CH3 H2O CH3CH(OH)CH2CH2CH2CH2CH3 R R R CH2C CH2C CH2C H H H R = (CH2)4CH3 13.102 a. CH2=CH(CH2)7CH3 10 C chain 1-decene b. CH2=CH(CH2)7CH3 c. CH2=CH(CH2)7CH3 d. polymerization: H2 CH3(CH2)8CH3 H2O CH3CH(OH)CH2CH2CH2CH2CH2CH2CH2CH3 R R R CH2C CH2C CH2C H H H R = (CH2)7CH3 13.103 cis-2-Hexene and trans-3-hexene are constitutional isomers because the double bond is located in a different place on the carbon chain (C2 vs. C3). CH3 CH2CH2CH3 C C H H CH2CH3 C C H cis-2-hexene CH3CH2 H trans-3-hexene © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part. Chapter 13–30 13.104 Determine the two monomer units for Saran. H Cl H Cl CH2 C CH2 C CH2 C CH2 C Cl Cl Cl Cl A B A B H CH2 C A Cl + CH2 C Cl Cl B © 2013 by McGraw-Hill Education. This is proprietary material solely for authorized instructor use. Not authorized for sale or distribution in any manner. This document may not be copied, scanned, duplicated, forwarded, distributed, or posted on a website, in whole or part.








