Experiment 11: Using a Nickel Spectrochemical Series to
advertisement

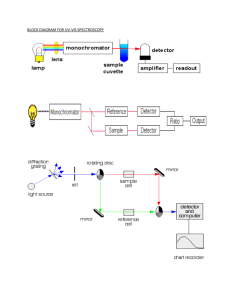
Experiment 11: Using a Nickel Spectrochemical Series to Derive Part of the d8 Tanabe-Sugano Diagram CH3500: Inorganic Chemistry, Plymouth State University Adapted in part from "d8 Energy Level Diagram" J Chem Ed, 45:94-97 (1968). Introduction: The energies of d-orbitals in free metal atoms and ions are degenerate, but this degeneracy is removed when the metal is bound to a ligand. The splitting of d-orbital energies in non-spherical environments is explained in Crystal Field Theory by treating the ligands as point charges and stating that the energies of d-orbtials rise in the presence of these point charges due to electron-electron repulsion. By simple electrostatic considerations, it follows that the energy of a d-orbital is influenced by 1) the magnitude of the charge of the ligand and 2) the proximity of the ligand to the orbital (which is in turn a combination of how well the orbital points at the ligand and the metal-ligand bond length). By considering the geometries only of the d-orbitals, it can be shown that in an octahedral compound the dx -y and dz orbitals (eg set) will rise to higher energy than the dxy, dyz, and dxz (t2g set). The difference in energy between the upper and lower energy levels is called the "Ligand Field Splitting Parameter" and is designated ΔO ("O" for Octahedral). The magnitude of ΔO is governed by the magnitude of the charges of the ligands and the metal-ligand bond distance. In other words, according to Crystal Field Theory and electrostatic considerations, more highly charged ligands and smaller ligands (decreased bond length) should result in a larger ΔO. Analysis of numerous octahedral compounds has empirically determined the effect of various ligands on the magnitude of the ligand field splitting parameter. This "Spectrochemical Series" is thus empirically derived and generally follows the order: I– < Br– < S2– < SCN– < Cl– < NO2– < N3- < F– < OH– < C2O42– < O2- < H2O < NCS– < CH3CN < py < NH3 < en < bipy < phen < NO2– < PPh3 < CN– < CO In other words, iodide results in a smaller ΔO than bromide and so forth. In some cases, this series matches Crystal Field Theory predictions (for example, bromide is a smaller ion than iodide, so it is expected to result in a larger ΔO). In many cases, however, it does not (for example, the carbonyl generally results in the largest ΔO although it is a neutral ligand), and thus the Spectrochemical Series stands as experimental evidence of the fundamental flaws in Crystal Field Theory. Although fundamentally flawed, Crystal Field Theory is conceptually simple and agrees well with experiments in certain circumstances. Therefore, it is a useful tool as long as its limitations are kept in mind, and it is applied only when appropriate. Experimentally, the value for ΔO can be found using UV-Vis spectroscopy. In the most simple systems, this is conceptually easy: an electron in a lower energy level (t2g) absorbs a photon with energy Eλ = hν and is promoted to the higher level (eg). The difference in energy between these levels is equivalent to both the energy of the photon (Eλ) and the ligand field splitting parameter (ΔO). In multielectron systems, however, an electron does not simply move between orbitals, but rather, the system moves between energy states. The interpretation of molecular UV-Vis spectra is therefore considerably confounded. A set of useful tools for relating states to spectra are the Tanabe-Sugano Diagrams, which plot the energies of the states versus the ligand field splitting parameter. In this lab, you will synthesize a series of d8 octahedral nickel compounds in solution using different ligands. These compound solutions will then be analyzed by UV-Vis spectroscopy to determine your own Spectrochemical Series, measure the energies of absorbed photons, and derive your own Tanabe-Sugano Diagrm for octahedral d8 systems. 2 2 2 © Copyright Plymouth State University and Jeremiah Duncan. May be distributed freely for education purposes only. 1 Safety Considerations: • Ammonia and ethylenediamine have foul odors and are harmful to inhale. Work with these compounds in a fume hood. • Properly dispose inorganic species (e.g. nickel) in the Inorganic Waste container. Procedure • Your group will be assigned to synthesize ONE of the compounds. Each group's data will be pooled at the end of class. • Remember to record what you do (including exact masses weighted out), data collected, and observations at each step in the procedure. A. Preparation of Compounds Ni(bipy)32+, Ni(en)32+, Ni(NH3)62+, Ni(OH2)62+ 1. You will be supplied with a 0.1M solution of NiSO4 (exact concentration will be given in lab). Use a volumetric transfer pipette to put 5mL of this solution into a 10 mL volumetric flask. 2. Make up your solutions as below a) 2,2-Bipyridine: dissolve 0.25 g in ~ 4mL of a 50/50 solution of ethanol and water. Use a stir bar to stir for up to five minutes. You may need to heat very gently to get it in solution. Add this to the volumetric flask and fill to the line with H2O. b) Ethylenediammine: add 200 μL to the flask using an autopipette. Fill to the line with H2O. c) Amine: dissolve 0.264g (NH4)2SO4 in ~2.5mL of water. Add 0.5 mL of 1.0M NaOH to this solution. Add the solution to the flask, then fill to the line with concentrated NH4OH. 3. Mix solutions well. Ni(DMSO)62+ 1. Dissolve 0.26g NiSO4·H2O in DMSO up to the line in a 10 mL volumetric flask. Calculate the molar concentration and report this with the rest of your results (see below). B. UV-Vis Analysis of Compounds 1. As soon as you have made your solution, take it to the UV-Vis spectrometer and take the spectrum from 300-1100 nm. Notes: a) The peaks should be well-separated. Some of them may exhibit shoulders--do not confuse the shoulder with one of the other peaks you are seeking. b) The two compounds with the smallest ΔO will have the lowest energy peak at a wavelength longer than detectable by the instrument. The compound with the largest ΔO will have the highest energy peak that is also undetectable. 2. Save your spectrum and export it to a CSV file. Save this file on a USB drive. 3. Take note of the wavelengths of the three lowest energy peaks. In some cases, one of these “peaks” may be a shoulder that is difficult to identify at first. If you have any trouble identifying your peaks, ask your instructor! 4. On another computer with internet access, import your spectrum into a spreadsheet. © Copyright Plymouth State University and Jeremiah Duncan. May be distributed freely for education purposes only. 2 5. Copy your full peaks and absorbances list to the Google document https://docs.google.com/spreadsheet/ccc? key=0AhR8E3EgztIhdEg0czdHRzl4MlRQRmQ0cC1rWVU4M2c 6. Make note of the wavelengths of the three lowest energy transitions in the Google document. 7. When all the other groups have uploaded their spectra, note the wavelengths of the three lowest energy transitions for each compound in your notebook. Also, copy all the spectral data into your spreadsheet. Analysis (Lab Notebook) The following must be completed in your lab notebook before you can turn in your Notebook Report: 1. Calculate frequencies of all three peaks for each compound in terms of wavenumbers (cm-1). 2. Construct a Tanabe-Sugano diagram for these three d-d transitions in the d8 systems. The four energy states from lowest to highest energy are 3A2, 3T2, 3T1, 3T1. Notes: 1) 3A2 is the ground state—the horizontal x-axis on your digram; 2) All energy transitions start from the ground state; and 3) the lowest energy transition is both ΔO and the difference in energies of the lowest excited state (3T2) and the ground state (3A2), thus the 3T2 line will be a straight line with slope=1. 3. Calculate the molar absorbtivity (ε) for the wavelength of the lowest energy peak for each compound (recall Beer's law: A = ε Cl ). Analysis and Conclusions (Lab Report) Your lab report is due by the time of your Final on Wednesday . Your report must be handed in BOTH electronically (via Moodle) and in hard copy form. See the document "InorgChemLabReportGuide.pdf" on the course website for guidelines on writing your report. In addition to the Analysis performed in your notebook, include the following in your report: 1. Convert the raw UV-Vis data for all the compounds from Absorbance vs Wavelength to Molar Absorbtivity vs Wavelength (you will almost certainly want to do this in a spreadsheet program!). Plot all four spectra on the same plot for inclusion in your report. Note: you should cut off the spectra of the bipy compound below about 450nm, so it does not throw off the scale of the overall plot. 2. Provide a table of the wavelengths and corresponding wavenumbers (ΔO in cm-1) for the three peaks in the spectra of each compound, and of the molar absorbtivities (ε) for the lowest energy. 3. Rank the ligands according to their ability to increase ΔO (i.e., what is your experimental Spectrochemical Series?). 4. Comment on your Spectrochemical Series. How is it consistent / inconsistent with the one given in the Introduction? How is it consistent / inconsistent with Crystal Field Theory? 5. Look back at the Ni(bipy)32+ compound. The large peak starting around 450nm is due to a metal-ligand charge transfer band. Compare the size and molar absorptivities of this peak with the d-d transitions you have otherwise been considering. Why is there such a huge discrepancy in the size of these peaks? © Copyright Plymouth State University and Jeremiah Duncan. May be distributed freely for education purposes only. 3