Cyclohexene Oxidation to Adipic Acid: Green Chemistry Protocol
advertisement

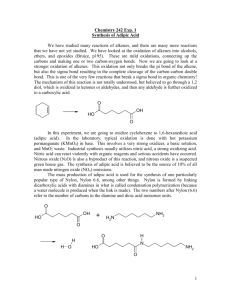
CHEM 321 Oxidation of cyclohexene to 1,6-hexanedioic acid Reed, S.M.; Hutchison, J.E. An Environmentally Benign Synthesis of Adipic Acid. J.Chem.Ed. 77, 2000, 1627-1629. Sato, K.; Aoki, M; Noyori, R. A Green Route to Adipic Acid: Direct Oxidation of Cyclohexenes with 30 percent Hydroxide Peroxide. Science. 281, 1998, 1646. Doxsee, K.M; Hutchison, J.E. In Green Organic Chemistry: Strategies, Tools, and Laboratory Experiments. Synthesis and Recrystallization of Adipic Acid. Brooks / Cole: United States. 2004; 135-141. The C=C double bond of alkenes offers many possibilities for transformation into other sorts of functional groups. One of these reactions is oxidation. Mild oxidation generates a vicinal diol, while harsher oxidation can completely cleave the C=C bond and make carbonyl groups out of the carbons formally in the double bond. The nature of the carbonyl group depends on substituents of the double bonded carbons in the reactant. For example, a cis or trans alkene can undergo vigorous oxidation to a dicarboxyclic acid. Many different oxidizing agents are available, but most of them are toxic and continue to be toxic once they have done their job. For example, the frequently used dichromate (Cr2O72-) becomes Cr3+, and both of these are toxic. This experiment is an example of an attempt at "green" chemistry because it uses more environmentally friendly reagents and creates little waste. The reaction is outlined below. The function of KHSO4 (essential for success) is probably to increase the oxidizing strength of H2O2 by making the reaction mixture acidic. The product of this reaction, 1,6hexanedioic acid or adipic acid, is used in the synthesis of Nylon-6,6. Nylon-6,6 is used in many common products including carpets, furniture, clothing, etc. WO42-, KHSO4 H2O2 + phase transfer catalyst O HO OH + H2 O O This reaction also illustrates the utility of a special kind of catalyst, a "phase transfer catalyst". Such compounds are constructed so that they can dissolve at effective concentrations in both aqueous and nonpolar solvents. They are said to be "amphiphilic". There are several types of phase transfer catalysts. The one chosen for this experiment is called Aliquat 336. It has a cationic "head" group at one end of an otherwise hydrophobic chain of atoms. Due to the positive charge of the "head" group, each molecule of catalyst requires a negatively charged counter-ion for electrical neutrality (chloride as Cl manufactured). These kinds of molecules dissolve to some extent in water because their polar head groups have a favorable enthalpy of interaction with polar water molecules. They also dissolve to some extent in nonpolar solvents because their long hydrophobic tails have numerous Van Der Waals interactions that balance ionic interactions. When these cations cross, or transfer, from water to a nonpolar phase, they "drag" with them whatever negative counter-ion is nearby. If that negative ion has chemical properties desired for the reaction (like, for example, being an oxidizing agent), then it can collide and perhaps react with a target compound in the nonpolar phase. Such an encounter and reaction would not otherwise be possible because the two needed reactants dissolve in different immiscible phases. N In this experiment, the tungstate ion (WO42-) is a good oxidizing agent. It is soluble in water, but it is insoluble in cyclohexene. The phase transfer catalyst drags it from its watery home into the cyclohexene, where it carries out the oxidation. The reduced form is still ionic and returns to the aqueous phase, where H2O2 reoxidizes it for another cycle. This means that only a small amount of tungstate is needed – it acts like a shuttle system to carry oxidizing power from aqueous H2O2 into the cyclohexene phase. In this way cyclohexene is gradually converted to hexanedioic (or adipic) acid. This product is soluble in hot water. Thus the two-phase system (aqueous = H2O2 + salts; organic = cyclohexene) gradually becomes a single, aqueous phase. Since the only chemicals that change are cyclohexene and H2O2, in principle the phase transfer catalyst and tungstate can be recovered and reused. To repeat the reaction one only needs to add more cyclohexene and H2O2. The physical properties of the reactants and products will be exploited to purify the adipic acid. Ions (salts, including carboxylate anions) are quite soluble in cool water but protonated (neutral) adipic acid is not. There are three parts to this purification process: (1) remove water-soluble reactants while mixture is acidic, leaving crude solid adipic acid (crystallization); (2) treat crude adipic acid with base to make it water soluble, and remove residual oily Aliquat 336 catalyst (alkalization); (3) recover adipic acid by acidifying the clear aqueous filtrate (acidification). Experimental protocol Caution: Wear gloves. Aliquat 336 is toxic, and 30 % - 35 % H2O2 causes severe burns: avoid skin contact. Preliminaries In order to maximize its solubility and chemical effectiveness, the phase transfer catalyst ("Aliquat 336™") will have been dissolved for you in cyclohexene. The solution will contain about 0.25 g of Aliquat 336 for every 2 g of cyclohexene (a total mass of 2.25 g). We can assume that the density of this solution is 0.8 g/mL. A convenient amount to oxidize is 2.0 g of cyclohexene, so a volume of 2.8 mL should contain the desired mass. Oxidation reaction In a 50 mL boiling flask, prepare a slurry of sodium tungstate (Na2WO4 2 H2O, 0.5 g), H2O2 (30% - 35%, 15 mL), and KHSO4 (0.4 g), and add a medium sized stir bar. None of the preceding quantities is critical, but don't skimp on H2O2. Add 2.8 mL of the cyclohexene/Aliquat 336 solution directly into the flask. Grease the joints of the condenser and mount it vertically on the apparatus. Reflux for at least 45 min while stirring rapidly (you may need to use a sand bath to get the stir bar to work properly). Watch the condenser closely to monitor the reflux. It is very important that the proper vapor level for reflux be maintained – too low and the reaction will not proceed; too high and cyclohexene will be lost through the top of the condenser. Workup Before starting, put a little water on ice to chill it for use in the workup. 1. CRYSTALLIZE THE CRUDE PRODUCT: While the reaction mixture is still hot, pour the liquid into a clean small beaker (cover beaker!). Do not worry if any of the yellow lower phase remains in the flask. Allow the contents of the beaker to cool, eventually on ice to 5 ºC. Adipic acid should precipitate - recover the solid by vacuum filtration on a Hirsch funnel. Adipic acid is slightly water-soluble, so you should use minimum amounts of water in all the following operations. 2. ALKALIZE THE CRUDE PRODUCT: Wash the beaker three times with 0.5 mL aliquots of ice-cold water and drizzle that water all over the crude product in the funnel to remove left-over salts. Return the solid from the funnel to the original beaker and add (slowly with stirring) just enough 6 M NaOH to dissolve all the small crystals. Observe carefully: any oily residue that accompanied the crude crystals will not dissolve, and you do not want to add too much NaOH (which is a water solution). When it looks like no more solid is dissolving, use bits of wide-range pH paper to check the pH. Try for a pH between 6 and 10. Don’t be hasty! Start with 2-3 mL of NaOH, and use a rubber policeman to stir in all solids. Be patient for them to dissolve! It might take anywhere from about 2 – 12 mL of NaOH. Once the pH is within this range, stir for a minute, then set aside while you prepare a thin pad of celite on top of a clean filter paper in the Hirsch funnel (suspend about a scoopula-end full of Celite in water to do this). The Celite will capture the oily residue. Clean and empty the filter flask. Filter the alkaline solution into the cleaned vacuum flask, saving the clear filtrate. Rinse the beaker once with 1 – 2 mL of water and pour this through the funnel, allowing it to mix with the previous filtrate. The beaker will be smeared with Aliquat 336 catalyst, which will need to be cleaned out later. 3. ACIDIFY THE CRUDE PRODUCT: Transfer the filtrate to a clean small beaker and add 6 M HCl with good stirring until it looks like no more precipitate is forming. Check the pH with a bit of wide-range paper. Continue adding 6 M HCl only until the pH is 2 or less. Cool the suspension on ice to 5 ºC, then vacuum filter on a clean Hirsch funnel to collect the final product. Coax remaining crystals into the filter with a rubber policeman. Drizzle a little ice water all over the surface of the solid to wash away most of the HCl. Analysis: Dry the crystals for a day, weigh, obtain a melting range, and run an IR spectrum using the ATR accessory. Cleanup: Filtrate from Workup step 1 goes in a bottle marked "Tungstate residues". The celite pad goes in the trash. To remove Aliquat 336 from glassware: o 1. The stir bar should still be in the boiling flask. Rinse these with small (~1 mL) amounts of hexanes, swirling for ½ min each time. Pour these washes into the Aliquat-smeared beaker. o 2. Add a small amount of acetone to the boiling flask with stir bar and swirl. Pour this wash into the Aliquat 336-smeared beaker. After swirling as long as it takes to clean the beaker, remove the stir bar, then pour the spent acetone / hexane mixture into the bottle marked “mixed organics”. o Finish washing glassware with detergent. When all tests on adipic acid are finished, put the product in the bottle marked "Adipic Acid Student Prep". LABORATORY REPORT: Minor (skeleton) report Keep the following in mind as you write this report: (a) The purposes for doing this experiment were not explicitly listed. Use your "chemistry common sense" to discover the main reasons for doing this experiment, and put them in your introduction. This should take no more than about 1-2 concise sentences. Hint: the main purpose was not to make adipic acid, nor to react cyclohexene. (b) The reaction as shown in this protocol is not balanced, but of course should be in your report (the “half-reaction” method is recommended). Show catalysts and recycled chemicals on the arrow. Use the balanced reaction to help you compute the theoretical yield. Guidelines for this report: Title Source Reference Introduction containing: o the experiment objective(s) (what was the purpose of performing the experiment?); o the context (the experiment that was performed as a means through which the purpose could be met – pertinent concepts and techniques included); o the rationale (why is this objective worthwhile?); and o Figure 1 should show just the above reaction, but balanced (no mechanism necessary this week). Use ChemDraw. Half reaction work need not be here, but should be shown in the Appendix. Experimental methods (refer to previous experiment handouts for examples) Results presenting actual data/observations that support whether the goal was met or not: o Sentences stating/introducing results / tables / figures o % yield (reported as g product and % of theoretical), o melting range, o IR spectra (with proper comparison compounds as additional figures), and o IR peak assignment table (with proper comparison compounds included). Discussion containing: o a reiteration of the objective, o comments on how these data compare to published values and what conclusions can be drawn, o plausible reasons for percent yield deviations from 100 percent, o chemistry concepts supporting results/interpretations (including figures when appropriate, such as reactions for chemical tests), and o a summary sentence indicating whether or not objectives were met References (and appropriate citations throughout the report) Appendix containing: o balanced reaction (with half reactions) work o % yield calculations (consider stoichiometry, remember that "x% H2O2" means that x% of the measured mass is H2O2 (the balance is water)) REMEMBER THESE GUIDELINES FOR ALL IR SPECTRA: Label and attach the IR spectra to your lab report, or insert them into the report document. Your RESULTS section should direct the reader to IR spectra (labeled as FIGURES): How do I label an IR spectrum? With an appropriate figure caption (see example) and figure number under the figure The correct name of the chemical, spelled out, is in the caption The molecular structure of the chemical (so the reader may see what functional groups are present – include on experimental product as well, with the structure of the expected product) Circle individual peaks you choose to discuss in your report, and tell what vibration is the likely cause of each peak. You need to assign enough significant peaks to allow the reader to see that the product is what it is claimed to be and is pure (or contaminated) as claimed in the report. Label each chosen peak explicitly with the name of the responsible molecular vibration. Examples: O-H stretch, sp2 C-H stretch, C=O stretch. These assignments should be chosen to confirm that the molecule is of the class you claim it to be. You should not use the sp3 C-H stretch unless it is a main significant signature peak. Figure caption includes a citation to a bibliographic citation in your REFERENCES section What are the IR spectra that I should include in my report? IR spectrum of experimental product Published IR spectrum of expected major product, citation included Published IR spectra of any potential contaminants or side products that would show characteristic IR peaks, citation(s) included To decide whether contamination from specific compounds is significant, evaluate the relative intensities of pertinent peaks in the product spectrum along with intensities of those peaks in the spectra of the starting materials and/or plausible side products. Where can I find published IR spectra? (These resources are on the course webpage under “Useful Links” as well.) Spectral Database for Organic Compounds, SDBS. URL: http://sdbs.db.aist.go.jp National Institute of Standards and Technology, NIST Chemistry WebBook. URL: http://webbook.nist.gov./ PRE-LAB QUESTIONS 1. During reflux, what are some things you can do to prepare / setup for the rest of the experiment? 2. What comparisons should be made to the experimental IR spectrum of the product to make a valid interpretation about its identity? 3. Walk through the flow chart of procedural steps provided on the next page. In the acidification step, explain why the desired product will crash out as a solid. OXIDATION OF CYCLOHEXENE TO FORM ADIPIC ACID – FLOWCHART & RATIONALE FOR PROCEDURAL STEPS Step Waste Generated & Removed Contents of Interest (containing the product!) Chemistry / how it works Reflux Reaction mixture Adipic acid Phase transfer catalyst (PTC) Salts H2O2 --- PTC, H2O2, and WO42- are preserved (recycled) so need to be removed at end. Cyclohexene is used up so is no longer present. Solid Crude adipic acid crystals PTC Filtrate (aqueous) Salts H2O2 Crude product is neutral: Aqueous NON-soluble Organic soluble REMOVED Organic phase doesn’t exist in this step, so product crashes out (is a solid) and the filtrate is waste. Solid PTC Celite pad Anion of product is charged: Aqueous SOLUBLE Organic NON-soluble Cool, crystallize, & filter Resulting in: Alkalize solid product & filter Resulting in: Filtrate (aqueous) Anion of adipic acid Water OH ions REMOVED Product is in aqueous filtrate (not a solid) and solid is waste. Acidify filtrate product & filter Resulting in: Dry product for a day Weigh, IR, melting range Solid Reprotonated pure adipic acid Filtrate (aqueous) Water Na+ ions Cl- ions Reprotonated product is neutral: Aqueous NON-soluble Organic SOLUBLE REMOVED Organic phase doesn’t exist in this step, so product crashes out (is a solid) and the filtrate is waste.