Hydrogen Peroxide Oxidation Kinetics Lab Report
advertisement
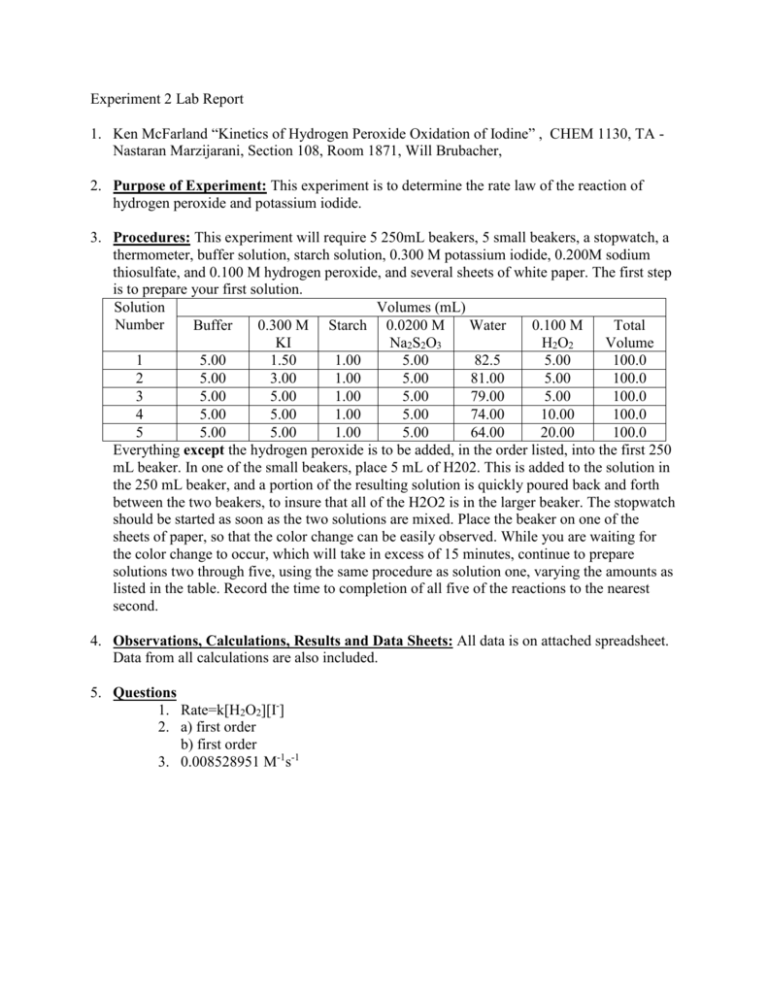
Experiment 2 Lab Report 1. Ken McFarland “Kinetics of Hydrogen Peroxide Oxidation of Iodine” , CHEM 1130, TA Nastaran Marzijarani, Section 108, Room 1871, Will Brubacher, 2. Purpose of Experiment: This experiment is to determine the rate law of the reaction of hydrogen peroxide and potassium iodide. 3. Procedures: This experiment will require 5 250mL beakers, 5 small beakers, a stopwatch, a thermometer, buffer solution, starch solution, 0.300 M potassium iodide, 0.200M sodium thiosulfate, and 0.100 M hydrogen peroxide, and several sheets of white paper. The first step is to prepare your first solution. Solution Volumes (mL) Number Buffer 0.300 M Starch 0.0200 M Water 0.100 M Total KI Na2S2O3 H2O2 Volume 1 5.00 1.50 1.00 5.00 82.5 5.00 100.0 2 5.00 3.00 1.00 5.00 81.00 5.00 100.0 3 5.00 5.00 1.00 5.00 79.00 5.00 100.0 4 5.00 5.00 1.00 5.00 74.00 10.00 100.0 5 5.00 5.00 1.00 5.00 64.00 20.00 100.0 Everything except the hydrogen peroxide is to be added, in the order listed, into the first 250 mL beaker. In one of the small beakers, place 5 mL of H202. This is added to the solution in the 250 mL beaker, and a portion of the resulting solution is quickly poured back and forth between the two beakers, to insure that all of the H2O2 is in the larger beaker. The stopwatch should be started as soon as the two solutions are mixed. Place the beaker on one of the sheets of paper, so that the color change can be easily observed. While you are waiting for the color change to occur, which will take in excess of 15 minutes, continue to prepare solutions two through five, using the same procedure as solution one, varying the amounts as listed in the table. Record the time to completion of all five of the reactions to the nearest second. 4. Observations, Calculations, Results and Data Sheets: All data is on attached spreadsheet. Data from all calculations are also included. 5. Questions 1. Rate=k[H2O2][I-] 2. a) first order b) first order 3. 0.008528951 M-1s-1