Chemistry 242 Exp. 1
Synthesis of Adipic Acid
We have studied many reactions of alkenes, and there are many more reactions
that we have not yet studied. We have looked at the oxidation of alkenes into alcohols,
ethers, and epoxides (Bruice, p195). These are mild oxidations, connecting up the
carbons and making one or two carbon-oxygen bonds. Now we are going to look at a
stronger oxidation of alkenes. This oxidation not only breaks the pi bond of the alkene,
but also the sigma bond resulting in the complete cleavage of the carbon-carbon double
bond. This is one of the very few reactions that break a sigma bond in organic chemistry!
The mechanism of this reaction is not totally understood, but believed to go through a 1,2
diol, which is oxidized to ketones or aldehydes, and then any aldehyde is further oxidized
to a carboxylic acid.
O
OH
HO
O
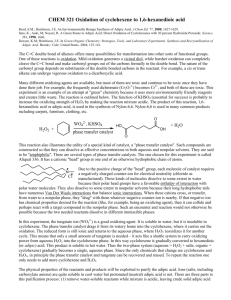
In this experiment, we are going to oxidize cyclohexene to 1,6-hexanedioic acid
(adipic acid). In the laboratory, typical oxidation is done with hot potassium
permanganate (KMnO4) in base. This involves a very strong oxidizer, a basic solution,
and MnO2 waste. Industrial synthesis usually utilizes nitric acid, a strong oxidizing acid.
Nitric acid can react violently with organic reagents and serious accidents have occurred.
Nitrous oxide (N2O) is also a byproduct of this reaction, and nitrous oxide is a suspected
green house gas. The synthesis of adipic acid is believed to be the source of 10% of all
man made nitrogen oxide (NOx) emissions.
The mass production of adipic acid is used for the synthesis of one particularly
popular type of Nylon, Nylon 6.6, among other things. Nylon is formed by linking
dicarboxylic acids with diamines in what is called condensation polymerization (because
a water molecule is produced when the link is made). The two numbers after Nylon (6.6)
refer to the number of carbons in the diamine and dioic acid monomer units.
O
OH
HO
+
NH2
H2N
O
O
H
H
H O
N
HO
NH2
O
1
O
H
O
N
N
H
O
further
reactions
H
N
N
H
O
n
We will examine an alternative oxidation method using a phase transfer catalyst
and hydrogen peroxide as the initial source of oxygen. This is a much milder
environment, less dangerous, and producing only water as a byproduct. The mechanism
is not known for sure, but it is suspected that the sodium tungstate (Na2WO4) is the active
oxidizing agent, being similar in structure to potassium permanganate. The tungstate
functions catalytically. It is replenished by the peroxide.
One problem of this reaction is that sodium tungstate, being ionic, has very
limited solubility in an organic medium while cyclohexene is insoluble in water.
Although the water/peroxide/sodium tungstate mixture and cyclohexene will remain
immiscible, we can expedite the mixture of reactants with each other using a phase
transfer caytalyst (Bruice p462 and p951). Quaternary ammonium ions having non-polar,
hydrophobic groups, while ionic, can be appreciably soluble in organic mediums as well.
These ammonium ions can pair with negative ions to make salts and carry the negative
ions into the organic solution. Thus, the ammonium salt carries the ions from one
immiscible liquid phase to another. Our phase transfer catalyst is called Aliquat 336
[(CH3CH2CH2CH2CH2CH2CH2CH2)3NCH3+ Cl-]. Aliquat 336 and sodium tungstate
function catalytically, so only the inexpensive hydrogen peroxide needs to be present in
stoichiometric amounts. Our phase transfer catalyst will actually be a mixture of aliquat
336 and potassium hydrogen sulfate.
O
OH
HO
O
2 R4NCl
(R4N)2WO4
R4N2 [reduced tunstate]
2 R4NCl
organic phase
aqueous phase
Na2WO4
2 NaCl
H2O
2 NaCl
Na2 [reduced tunstate]
H2O2
2
Techniques:
Reflux, recrystallization, phase transfer catalysis.
Safety Precautions:
Avoid contact with phase transfer catalyst as it is an irritant and can transport
contaminants through the skin. 30% hydrogen peroxide is a good oxidizer; avoid having
it touch your skin or clothing. Cyclohexene is flammable and can have a disagreeable
odor.
name of
compound
cyclohexene
m.w.
mL
used
density
(g/mL)
0.811
-
-
82.14
Na2WO4
2H2O
H2O2
(30% w/w)
Aliquat 336
329.9
KHSO4
136.2
-
-
Adipic Acid
146.1
n.a.
n.a.
34.0
1.11
404.2
0.884
Limiting reagent
grams
used
moles
used
n.a.
n.a.
ratio of moles
theory used purpose
product
________________________________________________
Reaction:
1.
2.
3.
4.
5.
Place 0.50 g of sodium tungstate dihydrate (Na2WO4 2 H2O) in a 50 mL round
bottom flask equipped with a stir bar and a water cooled condenser.
Add 0.5 g of Aliquat 336, which is an extremely viscous liquid and very difficult
to transfer. Weigh it directly into your reaction flask. Since it is catalytic, no
need to have exactly 0.50 g (remember sig figs?).
Add 11.98 g of 30% hydrogen peroxide and 0.37 g KHSO4.
Stir, then add 2.00g of cyclohexene. This order of addition of ingredients is
important.
Use a sand bath to heat mixture at a reflux for one hour. After the first half hour,
rinse down any cyclohexene in the condenser with a few mL of distilled water
using a pipette to prevent loss of reactant. It is important that the stirring be
brisk for the full hour to have efficient mixing of the two phases to help out the
phase transfer catalyst. Also, watch that the cylcohexene does not escape out the
top of the condenser. If it rises beyond the half way mark of the condenser, turn
down the heat. You may even need to temporarily remove the heat source
altogether if you heat too vigorously initially. The reaction is finished when
3
there are no longer two phases present. You may periodically stop the stirring
briefly to check if the reaction is done.
Isolation:
6. Transfer the reaction mixture to a 50 mL beaker, leaving behind any phase
transfer catalyst that may stick to the walls of the flask or form an oily layer on
the bottom of the flask. It is better to risk reduction of yield than accidentally
mix your product with the phase transfer catalyst that has separated out as this
will complicate the purification process.
7. Cool the beaker rapidly in an ice bath. A precipitate should form in 20 minutes.
Filter to isolate your product
8. Set some of this crude product aside to get a melting point after it has dried.
9. Recrystallize the crude product using hot water.
Characterization:
10. After drying for a week, take a mp of the crude and the recrystallized products.
Is there a difference? Why or why not?
1. What are the products of these oxidation reactions? (see end of chapter 19
for summary of oxidation reactions all in one place).
KMnO4, HCl
1. O3, -78 C
2. Zn, H2O
PCC
OH
CH2Cl2
4
2. Crown ethers can also be used as phase transfer catalysts. What is a crown
ether in general? Draw the structure of one and name it. How does a crown
ether work as a phase transfer catalyst? (Haven’t covered this yet? – a
glossary in a lecture text can be a wonderful aid).
3. Adipic acid can also be produced in several steps from 1, 5 hexadiene. Write
a multistep synthesis that shows how to do this and include the reaction
conditions and the structure of each intermediate in these steps.
References:
Organic Chemistry, P. Bruice, Pearson Prentice Hall, 2007
Green Organic Chemistry, K. Doxsee & J. Hutchison, Thomson Brooks/Cole, 2004
K. Sato, M. Aoki, and R. Noyori, “A ‘Green’ Route to Adipic Acid: Direct Oxidation of
Cyclohexenes with 30 Percent Hydrogen Peroxide,” Science, 1998, 281, p1646
5