Below is the structure of cortisone, part of
advertisement
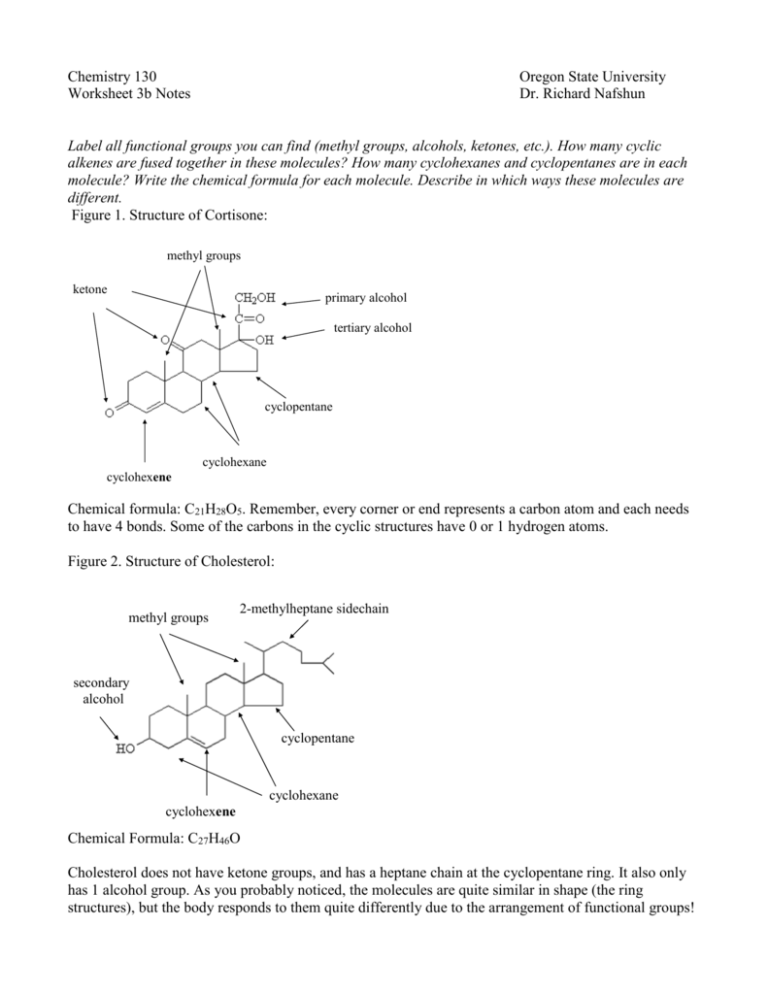
Chemistry 130 Worksheet 3b Notes Oregon State University Dr. Richard Nafshun Label all functional groups you can find (methyl groups, alcohols, ketones, etc.). How many cyclic alkenes are fused together in these molecules? How many cyclohexanes and cyclopentanes are in each molecule? Write the chemical formula for each molecule. Describe in which ways these molecules are different. Figure 1. Structure of Cortisone: methyl groups ketone primary alcohol tertiary alcohol cyclopentane cyclohexane cyclohexene Chemical formula: C21H28O5. Remember, every corner or end represents a carbon atom and each needs to have 4 bonds. Some of the carbons in the cyclic structures have 0 or 1 hydrogen atoms. Figure 2. Structure of Cholesterol: methyl groups 2-methylheptane sidechain secondary alcohol cyclopentane cyclohexane cyclohexene Chemical Formula: C27H46O Cholesterol does not have ketone groups, and has a heptane chain at the cyclopentane ring. It also only has 1 alcohol group. As you probably noticed, the molecules are quite similar in shape (the ring structures), but the body responds to them quite differently due to the arrangement of functional groups!