Chapter 21 Part 1 Wade 7th_CGD [Read
advertisement

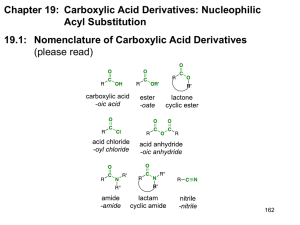
14.12.2011 Organic Chemistry, 7th Edition L. G. Wade, Jr. Chapter 21 Part 1: Structure and Properties of Carboxylic Acid Derivatives 2010, Prentice Hall Acid Derivatives All the derivatives can be converted to the carboxylic acid by acidic or basic hydrolysis. Esters and amides are commonly found in nature. Chapter 21 2 1 14.12.2011 Esters from Carboxylic Acids Esters can be made from the carboxylic acid through the Fischer esterification. Excess alcohol is used to drive the equilibrium toward the ester. Chapter 21 3 Nomenclature of Esters Esters are named as alkyl carboxylates. The first word is derived from the alkyl group of the alcohol, and the second word from the carboxylate group of the carboxylic acid. Chapter 21 4 2 14.12.2011 Cyclic Esters Reaction of —OH and —COOH on same molecule produces a cyclic ester called lactone. To name, add the word lactone to the IUPAC acid name or replace the -ic acid of common name with -olactone. Chapter 21 5 Amide Structure Amides are the product of the reaction of a carboxylic acid with ammonia or an amine. Not basic because the lone pair on nitrogen is delocalized by resonance. The C—N bond has double-bond character. Chapter 21 6 3 14.12.2011 Protonation of Amides Under acidic conditions, the double-bonded oxygen will get protonated. Chapter 21 7 Classes of Amides 3º amide 2º amide 1º amide 1° amide has one C—N bond (two N—H). 2° amide or N-substituted amide has two C—N bonds (one N—H). 3° amide or N,N-disubstituted amide has three C—N bonds (no N—H). Chapter 21 8 4 14.12.2011 Nomenclature of Amides O CH3 CH3CHC N CH2CH3 N-ethyl-N-methyl-2-dimethylpropanamide (N-ethyl-N-methylisobutyramide) CH3 For 1° amide, drop -ic or -oic acid from the carboxylic acid name, add -amide. Alkyl groups bonded to nitrogen are named with N-alkyl to indicate their attachment to the nitrogen atom. Chapter 21 9 Cyclic Amides Cyclic amides are called lactams. To name, add the word lactam to the IUPAC acid name or replace the -ic acid of common name with -olactam. Chapter 21 10 5 14.12.2011 Nitriles Nitriles contain the cyano group (—C≡N). They can be hydrolyzed to carboxylic acids. Chapter 21 11 Structures of Acetonitrile and Propyne In both compounds, the atoms at the ends of the triple bonds are sp hybridized, and the bond angles are 180°. In place of the acetylenic hydrogen atom, the nitrile has a lone pair of electrons in the sp orbital of nitrogen. The nonbonding electrons on the nitrogen are not basic. Chapter 21 12 6 14.12.2011 Naming Nitriles CN CH3 C N acetonitrile CH3 CH2 CH CH2 COOH CN cyclopropanecarbonitrile 3-cyanopentanoic acid For IUPAC names, add -nitrile to the alkane name. The Ethyl Octanoate group can also be named as a substituent, the cyano group. Common names come from the carboxylic acid. Replace -ic acid with -onitrile. Chapter 21 13 Acid Halides O O R C Cl R C Br acid chloride (acyl chloride) acid bromide (acyl bromide) Also called acyl halides. These are more reactive than carboxylic acids, so they are used to synthesize other acid derivatives such as esters and amides. Used in the Friedel–Crafts acylation to make acylbenzenes. Chapter 21 14 7 14.12.2011 Acid Halide Nomenclature O CH3CH2 C Cl propanoyl chloride Br CH3CHCH2 O C Br 3-bromobutanoyl bromide Named by replacing -ic acid with -yl halide. Acyl chlorides are more common. Chapter 21 15 Acid Anhydrides Two molecules of acid combine with the loss of water to form the anhydride. Anhydrides are more reactive than acids, but less reactive than acid chlorides. A carboxylate ion is the leaving group in nucleophilic acyl substitution reactions. Chapter 21 16 8 14.12.2011 Anhydride Nomenclature O CH3 O C O C CH3 ethanoic anhydride (acetic anhydride) O CF3 O O CH3 C O C CF3 trifluoroethanoic anhydride (trifluoroacetic anhydride) O C O C H ethanoic methanoic anhydride (acetic formic anhydride) The word acid is replaced with anhydride. For a mixed anhydride, name both acids. Chapter 21 17 Multifunctional Compounds The functional group with the highest priority determines the parent name. OH CH3 CH2 CH C N 2-hydroxybutanenitrile acid > ester > amide > nitrile > aldehyde > ketone > alcohol > amine > alkene > alkyne Chapter 21 18 9 14.12.2011 Boiling Points of Carboxylic Acid Derivatives Chapter 21 19 Intermolecular Forces of Amides Chapter 21 20 10 14.12.2011 Melting Points Amides have very high boiling points and melting points compared to other compounds of similar weight. Melting points increase with increasing number of N—H bonds. Tertiary amides cannot hydrogen bond, but still have high boiling points. Chapter 21 21 Solubility Acid chlorides and anhydrides are too reactive to be used with water or alcohol. Esters, 3° amides, and nitriles are good polar aprotic solvents. Solvents commonly used in organic reactions: Ethyl acetate Dimethylformamide (DMF) Acetonitrile Chapter 21 22 11 14.12.2011 Common Solvents Chapter 21 23 IR Spectroscopy Chapter 21 24 12 14.12.2011 IR Spectrum of Ethyl Octanoate Chapter 21 25 IR Frequencies of Lactones and Lactams • Ring strain in lactones and lactams increases the carbonyl stretching frequency. Chapter 21 26 13 14.12.2011 IR Spectrum of Propionic Anhydride Chapter 21 27 Typical 1H–NMR Absorptions Chapter 21 28 14 14.12.2011 NMR Spectra of DMF Chapter 21 13C–NMR 29 Spectroscopy The carbonyl carbons of acid derivatives appear at shifts around 170 to 180 ppm, slightly more shielded than the carbonyl carbons of ketones and aldehydes. The α-carbon atoms absorb around 30 to 40 ppm. Chapter 21 30 15