Amides Logan,Tristan,Sean
advertisement

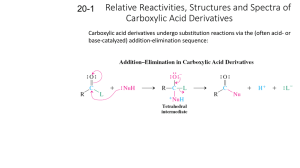
Tristam, Sean, Downey Mr.Snyder Dec 04/13 General Structure Amides are derived from carboxyclic acids and amines 1st degree amides only have 1 carbon chain 2nd degree amides have two carbon chains: one off of the nitrogen and one off of the main 3rd degree amides have three carbon chains: the main, and two off of the nitrogen Steps In naming amides • First step identify the parent chain starting from the carbonyl group • Second step is to replace the ending by taking the “e” off and replacing it with amide • Third step is naming in methyl or ethyl groups off the parent chain which are named the same way as alkanes or alkenes unless the methyl ethyl or propyl comes off the nitrogen which then gives the name N in front of the methyl or ethyl. Also if two come off the nitrogen it is nn dimethyl, ethyl etc • Fourth step in the case of a double or triple bond the same rule applies just like alkanes, alkenes and alkynes Examples ( name amide) O N O N Can you draw these amides 1,3,5-triethyl-N-methyl-N-propyloctanamide 2,4-diethyl-1,3,5-trimethly-N,N-dipropyl-cis-7-nonenamide Uses uses of amides include coloring for the dye industry, a number of different neurotransmitter drugs, treatment of natural gas, explosives, nylon, Kevlar, latex, latex thickeners, adhesives, paint, cosmetics, and paper pigment. Properties Amides are neutral compounds -- in contrast to their seemingly close relatives, the amines, which are basic. The amide linkage is planar-- even though we normally show the C-N connected by a single bond, which should provide free rotation. The solubility's of amides and esters are roughly comparable. Typically amides are less soluble than comparable amines and carboxylic acids since these compounds can both donate and accept hydrogen bonds. Amide Reactions Formation Carboxylic acids react with Ammonia(NH 3) or a 1° or 2° Amine compound This is a condensation reaction which results in the formation of a H 2O and an Amide compound Formation Cont’d Seen above a carboxylic acid reacts with a 1° Amine If a 2° or Ammonia are used it is a very similar reaction with a slightly different product amine Formation Conditions For condensation to occur there are two factors which are to be met: Heat Reaction takes place in conc. H 2SO4 Other Amide Reactions There is one other reaction which breaks Amides into a Carboxylic acid and a 1°, 2° amine or an Ammonia known as Hydrolysis Essentially the reverse of Condensation as water is reacted with an Amide to produce The only condition for this reaction is the presence of an acid or base as a catalyst Bibliography http://en.wikipedia.org/wiki/Amide http://www.chemguide.co.uk/organicprops/amides/backg round.html http://www.ask.com/question/uses-of-amides http://www.ivy-rose.co.uk/Chemistry/Organic/NamingAmides.php