Chapter 21 Carboxylic Acid Derivatives
advertisement

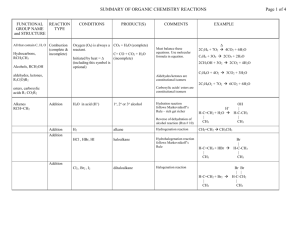
Organic Chemistry, 6th Edition L. G. Wade, Jr. Chapter 21 Carboxylic Acid Derivatives Acid Derivatives • All can be converted to the carboxylic acid by acidic or basic hydrolysis. • Esters and amides common in nature. => Chapter 21 2 Naming Esters • Esters are named as alkyl carboxylates. • Alkyl from the alcohol, carboxylate from the carboxylic acid precursor. O CH3CH2 OH O + H + HO C CH3 CH3CH2 O C CH3 + H2O ethanol ethanoic acid ethyl ethanoate ethyl alcohol acetic acid ethyl acetate => Chapter 21 3 Name These CH3 O O HCOCH2 CH3CHCH2OCCH3 isobutyl acetate 2-methylpropyl ethanoate benzyl formate benzyl methanoate => Chapter 21 4 Cyclic Esters • Reaction of -OH and -COOH on same molecule produces a cyclic ester, lactone. • To name, add word lactone to the IUPAC acid name or replace the -ic acid of common name with -olactone. O H3C O 4-hydroxy-2-methylpentanoic acid lactone -methyl--valerolactone CH3 => Chapter 21 5 Amides • Product of the reaction of a carboxylic acid and ammonia or an amine. • Not basic because the lone pair on nitrogen is delocalized by _resonance. O C H O N H H C + H N H H Bond angles around N are close to 120. Chapter 21 6 => Classes of Amides • 1 amide has one C-N bond (two N-H). • 2 amide or N-substituted amide has two C-N bonds (one N-H). • 3 amide or N,N-disubstituted amide has three C-N bonds (no N-H). => Chapter 21 7 Naming Amides • For 1 amide, drop -ic or -oic acid from the carboxylic acid name, add -amide. • For 2 and 3 amides, the alkyl groups bonded to nitrogen are named with Nto indicate their position. O CH3 CH3CHC N N-ethyl-N,2-dimethylpropanamide CH2CH3 N-ethyl-N-methylisobutyramide CH3 Chapter 21 8 => Cyclic Amides • Reaction of -NH2 and -COOH on same molecule produces a cyclic amide, lactam. • To name, add word lactam to the IUPAC acid name or replace the -ic acid of common name with -olactam. O N H CH3 4-aminopentanoic acid lactam -valerolactam => Chapter 21 9 Nitriles • -CN can be hydrolyzed to carboxylic acid, so nitriles are acid derivatives. • Nitrogen is sp hybridized, lone pair tightly held, so not very basic (pKb about 24). => Chapter 21 10 Naming Nitriles • For IUPAC names, add -nitrile to the alkane name. • Common names come from the carboxylic acid. Replace -ic acid with -onitrile. Br C N CH3CHCH2CH2CH2CN 5-bromohexanenitrile -bromocapronitrile Cyclohexanecarbonitrile => Chapter 21 11 Acid Halides • More reactive than acids; the halogen withdraws e- density from carbonyl. • Named by replacing -ic acid with -yl halide. O C Br Cl O CH3CHCH2C Br 3-bromobutanoyl bromide -bromobutyryl bromide => benzoyl chloride Chapter 21 12 Acid Anhydrides • Two molecules of acid combine with the loss of water to form the anhydride. • Anhydrides are more reactive than acids, but less reactive than acid chlorides. • A carboxylate ion is the leaving group in nucleophilic acyl substitution reactions. O R C O H O O H O C R O R C O C R => Chapter 21 13 Naming Anhydrides • The word acid is replaced with anhydride. • For a mixed anhydride, name both acids. • Diacids may form anhydrides if a 5- or 6membered ring is the product. O O O C O C CH3 O ethanoic anhydride acetic anhydride O CH3 1,2-benzenedicarboxylic anhydride phthalic anhydride => Chapter 21 14 Multifunctional Compounds • The functional group with the highest priority determines the parent name. • Acid > ester > amide > nitrile > aldehyde > ketone > alcohol > amine > alkene > alkyne. O C OCH2CH3 ethyl o-cyanobenzoate => CN Chapter 21 15 Boiling Points Even 3 amides have strong attractions. Chapter 21 => 16 Melting Points • Amides have very high melting points. • Melting points increase with increasing number of N-H bonds. m.p. -61C m.p. 28C m.p. 79C => Chapter 21 17 Solubility • Acid chlorides and anhydrides are too reactive to be used with water or alcohol. • Esters, 3 amides, and nitriles are good polar aprotic solvents. • Solvents commonly used in organic reactions: Ethyl acetate Dimethylformamide (DMF) Acetonitrile Chapter 21 =>18 Interconversion of Acid Derivatives • Nucleophile adds to the carbonyl to form a tetrahedral intermediate. • Leaving group leaves and C=O regenerates. => Chapter 21 19 Reactivity Reactivity decreases as leaving group becomes more basic. => Chapter 21 20 Interconversion of Derivatives More reactive derivatives can be converted to less reactive derivatives. => Chapter 21 21 Acid Chloride to Anhydride • Acid or carboxylate ion attacks the C=O. • Tetrahedral intermediate forms. • Chloride ion leaves, C=O is restored, H+ is abstracted. _ => O O O R' C O H R C O - H+ Cl R H C +O Cl R C O O C R' + HCl C R' O Chapter 21 22 Acid Chloride to Ester • Alcohol attacks the C=O. • Tetrahedral intermediate forms. • Chloride ion leaves, C=O is restored, H+ is abstracted. => _ O R' O H R C O Cl R C Cl +O H R' Chapter 21 O - H+ R C O R' + HCl 23 Acid Chloride to Amide • Ammonia yields a 1 amide • A 1 amine yields a 2 amide • A 2 amine yields a 3 amide => Chapter 21 24 Anhydride to Ester • Alcohol attacks one C=O of anhydride. • Tetrahedral intermediate forms. • Carboxylate ion leaves, C=O is restored, H+ is abstracted. => Chapter 21 25 Anhydride to Amide • Ammonia yields a 1 amide • A 1 amine yields a 2 amide • A 2 amine yields a 3 amide => Chapter 21 26 Ester to Amide • Nucleophile must be NH3 or 1 amine. • Prolonged heating required. Surprise! => Chapter 21 27 Leaving Groups A strong base is not usually a leaving group unless it’s in an exothermic step. => Chapter 21 28 Transesterification • One alkoxy group can be replaced by another with acid or base catalyst. • Use large excess of preferred alcohol. Chapter 21 29