Flame test lab
advertisement

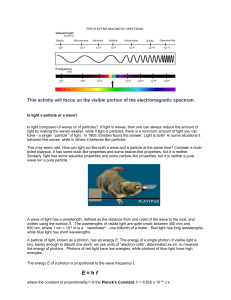
Flame Test Lab You will be conducting flame tests to see the different colors emitted by substances and then use what you have observed to identify an unknown substance. SAFETY: You must wear googles. Hair should be tied back and loose articles of clothing should be restrained. Any objects that have been burned should be doused with water before throwing into the trash. Procedure: 1. Obtain a beaker with distilled water. 2. Obtain cotton swabs. 3. Take one of the beakers of labeled substances to your lab station. 4. To light your burner. Hook the rubber tubing to the gas and the burner. Have your flint ready (or match lit) and turn on the gas. If using a match, slowly raise the match from the bottom of the burner to the top. Adjust the oxygen ring until you have a blue flame. 5. Dip the cotton swab in the water, then into the substance. 6. Hold the cotton swab in the flame and note the color. 7. Put the cotton swab in water then dispose in the trash. 8. Repeat with the other substances including the unknown. Name Formula of Metal Chloride Color of Flame Approximate Wavelength Lithium chloride ;Sodium chloride Barium chloride Potassium Chloride Strontium chloride Copper chloride Unknown Representative Wavelength Color Wavelength Region (nm) (nm) Violet 420 400 - 440 Blue 455 440 - 470 Blue480 470 - 490 green Green 525 490 - 560 Yellow 565 560 - 570 -green Yellow 580 570 - 585 Orang 620 585 - 630 e Red 660 630 - 700 Analysis Questions 1. Most salts contain a metal and a non-metal. Look at the compounds we tested and determine whether it is the metal or the non-metal that is responsible for the color produced in the flame test for that salt. How can you be sure your answer is correct? 2. What colors did the unknown produce in the flame? What is the unknown? 3. Could flame tests be useful in determining identities of metals in a mixture of two or more salts? If so, what problems might arise? If not, why not? Explain your answer. 4. How sensitive is the flame test? Could it be used to accurately identify metal ions? What difficulty could there be when identifying ions by the flame test? 5. Fireworks come in a variety of colors. Explain how the fireworks manufacturers could get these colors. Hints for your lab report Introduction: You might have to look online to get some information about this topic. Think about your procedure, what problem are you trying to solve and how are you trying to solve it? Think about what background information both you and your reader would have to know to understand what you will base your conclusions on. Conclusion: Since you haven’t learned much about this topic, it is really important that you talk about what this experiment makes you curious about and what you think you might need to know to get accurate results.