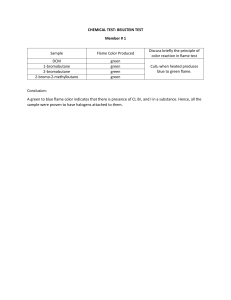
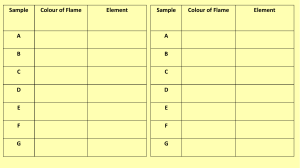
Flame Test Virtual Lab To complete this lab please visit: http://www.mrpalermo.com/virtual-labspectroscopy.html Paste or copy the following information into your lab notebook. Using Part 2: Flame Test you will view online visuals (found at the above url) to help you fill out the following data table and identify 3 unknown elements. In addition to the above compounds determine the color flame produced by the following elements: Element Flame Color Strontium Copper Lead Magnesium Zinc Resources: http://www.stevespanglerscience.com/lab/experiments/flame-test/ http://www.chemguide.co.uk/inorganic/group1/flametests.html https://www.acs.org/content/dam/acsorg/about/governance/committees/chemicalsafet y/safetypractices/flame-tests-demonstration.pdf Analysis Questions: Explain how colors in the flame test are produced. How are the electrons “excited” in Part 2 of the experiment?? What does it mean when the electrons are “excited”? Explain why we did not see distinct lines (like the emission spectrum in Part I.) when the metal salts were burned. Aerial fireworks contain gunpowder that produce colors. What elements would you include to produce the following colors? Red Orange Green