Experiment 5. The C Isomerization, Addition and Oxi Chemistry of
advertisement

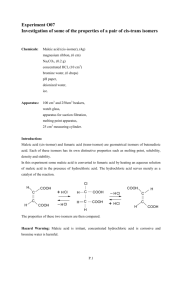
Experiment 5. The C Chemistry of Alkenes: Cis-Trans Trans Isomerization, Addition and Oxidation Reactions. References: Brown & Foote, Chapters 2, 2,and 6-8 INTRODUCTION: In this experiment you will investigate some of the common alkene reactions. In Part A, you will carry out a cis-trans trans isomerization. In Part B, you will examine some addition reactions and Part C will involve an oxidation. BACKGROUND: Figure 1: Reactions of Alkenes Part A: Cis-Trans Trans Isomerization of Maleic Acid. Maleic acid and fumaric acid have the same molecular formula, C4H4O4, but different structures. They are known as the cis and trans isomers respectively, and have widely different physical, physi chemical and biochemical properties. As shown below, the carboxylic groups in maleic acid lie on the same side of the double bond [[cis- or (Z)-isomers]; isomers]; the carboxylic groups in fumaric acid lie on opposite sides of the double bond [[trans- or (E)-isomers]. ers]. Using a strong acid catalyst coupled with a strong nucleophile (such as HCl), maleic acid can be converted to fumaric acid, as shown below. The reaction proceeds via a conjugate 1,4 addition followed by a rotation and then the loss of HCl (reverse of the 1st step). Part B: Addition to Alkenes. There are a number of reagents that may be added to an alkene, some with different mechanisms (see Solomons & Fryhle, Ch.8). However, the final products can fall into two categories, syn and anti additions, as shown in Figure 1. You should make sure you understand the addition reactions. In Part B, you will briefly examine the Radical Halogenation of alkanes (see Solomons & Fryhle, Ch.10), so you should also make yourself familiar with Radical Hal Halogenation. In today’s experiment we will use cyclohexene as a typical alkene, ligroin (also known as petroleum ether which is mostly hexanes) boiling at 66 66-75 oC as a typical mixture of alkanes, and unpurified ligroin as an impure alkane. Part C: Reduction/Oxidation of Alkenes Alkenes. Alkenes can either be reduced to an alkane or oxidized. In this experiment we will examine an oxidation reaction. Our oxidant in this experiment will be potassium permanganate and it will follow this general equation: There re are also oxidation reactions which result in the cleavage of the C=C and the products are some type of carbonyl or carboxyl functional group (depending on the oxidant and reactant), but we will not be examining these O Oxidative Cleavages in this experiment. PRE-LAB LAB PREPARATION PREPARATION: Read the experimental procedure so that you are prepared for the lab and you understand the safety and disposal information for the chemicals you are using in this experiment. 1. Which would you expect to be more thermodynamic thermodynamically ally stable, fumaric acid or maleic acid? Why? 2. Write general equations for reactions used in Parts B & C (synthesis not mechanisms). Use generic functional groups (R1, R2 etc)) for these reactions, like in the background section. 3. Draw the products of bromination of Z- and E-2-butene. Specify which (if any) of these products is optically active. Are any of the structures identical? EXPERIMENTAL PROCEDURE: Start with Part A and perform Parts B, C and D as time permits during the lab (ie. during the 30 min reflux). You should finish all Parts by the end of the lab. All work MUST be done individually. Safety and Disposal Data for Compounds used in Experiment 4. Compound Mol. Wt. (g/mol) Safety and Disposal Data Bromine 159.80 Very toxic. Corrosive. Causes severe burns. Use gloves when handling. Dispose in Halogenated Organic Waste. Chloresterol 386.66 Avoid contact with skin and eyes. Chlorofrom 119.37 Harmful if swallowed. Irritating to skin. Dispose in Halogenated Organic Waste (Contains Br2). Cyclohexane 84.16 Highly flammable. Harmful. Irritating to skin. Dispose in Halogenated Organic Waste (Contains Br2). Cyclohexene 82.14 Highly flammable. Harmful in contact with skin and if swallowed. Dispose in Halogenated Organic Waste (Br2). Fumaric Acid 116.07 Irritant. Dispose in Solid Waste. Gasoline 2M & 6M (HCl) Hydrochloric Acid Ligroin --- Highly Flammable. Irritant. Harmful if swallowed. Dispose in Halogenated Organic Waste (Contains Br2). 36.46 Irritating to eyes, respiratory system and skin. Use gloves, especially when handling 6M HCl. Dispose HCl in ‘Inorganic Acids and Salts’. --- Highly Flammable. Dispose in Halogenated Organic Waste. Maleic Acid 116.07 Irritant. Dispose in Solid Waste. Pinene 136.23 Flammable. Irritating to eyes, respiratory system and skin. Dispose in Halogenated Organic Waste (Contains Br2). Potassium Permanganate (in 10% H2SO4) 158.03 Strongly Corrosive!! Oxidizing. Harmful if swallowed. Dispose in ‘Inorganic Acids and Salts’ Equipment Used All the equipment needed to perform this experiment should be in one of the three drawers in the top row of your workstation, one of the drawers underneath your fumehood or the common counter for your TAs group. However the microkit (microscale glassware) should be obtained directly from your TA. At the end of the experiment, return your complete CLEAN! microkit and get the TA to initial the return of your microkit. Part A: Cis-Trans Isomerization of Maleic Acid. 1. Refluxing Maleic Acid WEAR GLOVES!!! a) You should begin the experiment by washing the vial, the condenser AND the blue connector. If the previous person to use the equipment didn’t rinse it, there may be concentrated HCl on the equipment. b) Dissolve 1g of maleic acid in 1mL of warm water in a 10 mL cylindrical vial. Add 2 mL of conc. HCl and a boiling chip. c) Attach a micro-condenser to the vial, and then attach the condenser to a water supply (water goes in through the bottom, out through the top). Turn on the water supply, so that water is trickling out the exit tubing. d) Commence heating using the aluminum block, placed directly on top of the heater-stirrer. e) Once the solution starts to boil, the vapour will condense and fall back into the vial, known as refluxing. The reaction should reflux for 30 minutes. Once a good stable reflux is established, take the time to start the other parts (just keep an eye on the time!) 2. Collecting and Purifying the Fumaric Acid a) Cool the mixture to room temperature and then in an ice-water bath. b) Vacuum filter the crystals using 2 pieces of filter paper on a Hirsch funnel. Use the filtrate to rinse the cylindrical vial. BE CAREFUL! You are filtering ACID! If you don’t use more than one piece of filter paper, the acid eats through it and you will not collect any crystals. c) Recrystallize your Fumaric acid from 2M HCl (heat ~25 mL). If you forget how to recrystallize, refer to Exp. #3. Do not heat the solution very hot during the recrystallization! d) Dry the product as thoroughly as possible and weigh it. e) Calculate the % yield of your purified product. f) Determine the melting point of the starting Maleic Acid and your Fumaric Acid product. The melting point of one of the isomers is ~150°C above the melting point of the other isomer. So once your first isomer melts you may want use the Heat Booster on the Melting Point Apparatus. g) Run an IR of your Fumaric Acid to verify that you still have carboxylic acid groups and that you have not reacted and modified some of the functional groups or added new ones. SAFETY NOTE: Since you were using concentrated HCl, please rinse the glassware and connectors, so you don’t burn the next person to use the equipment. Part B: Addition to Alkenes. Record your results in a table format. Make good/brief observations! 1. Addition of Bromine (in Chloroform) a) Put two 1 mL portions of purified ligroin in two test tubes, 1 mL of unpurified ligroin in a third and 1 mL of cyclohexene in a fourth. b) Add 5-6 drops of a 3% solution of bromine in chloroform into each test tube (one at a time). c) If decolourization occurs, test for the evolution of hydrogen bromide (due to Radical Halogenation) with wet litmus. d) Place one of the purified ligroin-containing tubes in your locker out of the light (AS SOON AS you add the bromine!) and expose the other to bright sunlight or hold it close to a light bulb. If decolourization occurs, test for hydrogen bromide, as before. When a change is noted in the sample exposed to light, compare its appearance with that of the mixture kept in the dark. 2. Addition of Bromine-Water a) Measure 3 mL of a 3% aqueous solution of bromine into each of three test tubes. b) Add 1 mL portions of purified ligroin to the first test tube, 1 mL of unpurified ligroin to the second and 1 mL of cyclohexene to the third. c) Stopper each test tube and shake gently to transfer some bromine-water to the organic phase. Part C: Reduction/Oxidation of Alkenes. 1. Oxidation using Acid Permanganate a) Put 1 mL portions of purified ligroin in a test tube, 1 mL of unpurified ligroin in a second and 1 mL of cyclohexene in a third. b) Add permanganate solution (1% potassium permanganate in 10% sulfuric acid) dropwise until a purple color persists. Do you observe a difference in behaviour among the substances under test? Part D: Test for Unsaturation. The above, simple reactions (Parts B1, B2 & C1) can be used as a ‘test’ to differentiate alkanes from alkenes or to detect the presence of alkenes in alkanes. Use one of these ‘tests’ to determine which of the following hydrocarbons are saturated and which contain unsaturated material. Use approx. 1 mL of each trial compound and rank them from most to least saturated. Pinene, the principal constituent of turpentine oil Gasoline Cyclohexane Cholesterol (a 10% solution in CHCl3 was made for the tests). Note: Since this is a problem based lab, you will be required to include a brief conclusion of your findings.