Experiment O07
advertisement

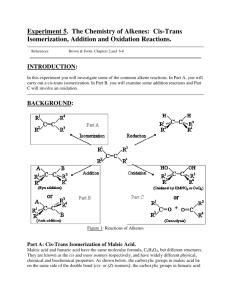
Experiment O07 Investigation of some of the properties of a pair of cis-trans isomers Chemicals: Maleic acid (cis-isomer), (4g) magnesium ribbon, (6 cm) Na2CO3, (0.2 g) concentrated HCl, (10 cm3) bromine water, (6 drops) pH paper, deionized water, ice. 100 cm3 and 250cm3 beakers, Apparatus: watch glass, apparatus for suction filtration, melting point apparatus, 25 cm3 measuring cylinder. Introduction: Maleic acid (cis-isomer) and fumaric acid (trans-isomer) are geometrical isomers of butenedioic acid. Each of these isomers has its own distinctive properties such as melting point, solubility, density and stability. In this experiment some maleic acid is converted to fumaric acid by heating an aqueous solution of maleic acid in the presence of hydrochloric acid. The hydrochloric acid serves merely as a catalyst of the reaction. Cl H COOH C + H Cl C H COOH H Cl COOH H H C COOH H Cl C H C COOH + HCl C H H COOH The properties of these two isomers are then compared. Hazard Warning: Maleic acid is irritant, concentrated hydrochloric acid is corrosive and bromine water is harmful. P.1 Experiment O07 Investigation of some of the properties of a pair of cis-trans isomers Procedure: A. Conversion of maleic acid to fumaric acid 1. Weigh out about 4 g of maleic acid in a clean dry 100 cm3 beaker. Add 10 cm3 of deionized water and warm slightly to dissolve the acid. 2. Add 10 cm3 of concentrated hydrochloric acid, and cover the beaker with a watch glass. Place the beaker inside a 250 cm3 beaker which is about one third full of water. Heat this water bath to boiling for about 5 minutes or until a solid material forms in the small beaker. 3. Cool the solution to room temperature by placing the small beaker with its contents in a cold water bath or in an ice bath. 4. Filter the reaction mixture by suction using the following set-up: 5. Stop suction, either by lifting the funnel or by disconnecting the tubing, and soak the residue in about 1 cm3 of cold water. (If you turn off the tap, you may get a 'suck-back' of water.) 6. Resume suction and dry the crystals by drawing air through them for a few minutes. 7. Transfer the crystals into a weighed watch glass and dry in an oven at about 120oC for 10 minutes. 8. Weigh the dried crystals of fumaric acid. P.2 Experiment O07 Investigation of some of the properties of a pair of cis-trans isomers B. 1. Comparison of properties of the two isomers Solubility in water: place about 1 g of each isomer into 10 cm3 of water in separate test tubes, shake to help dissolving. See which isomer is more soluble. 2. Melting point: using the electrical melting point apparatus, measure the melting point of the two isomers. 3. Acid strength: for each of the two isomers, prepare a solution by dissolving about 0.1 g of the compound in about 20 cm3 of distilled water. Divide the solution into 3 portions, one for each of the following tests: 4. a. Measure the pH of the solution. b. Add a 3 cm strip of magnesium ribbon. c. Add a small amount of solid sodium carbonate. Reaction with bromine water: suspend about 0.1 g of the acid in about 5 cm3 of water. Add 3 drops of bromine water to the resulting solution/suspension. Shake and observe. P.3 Experiment O07 Investigation of some of the properties of a pair of cis-trans isomers Name: Seat No.: Date: Grade: Results: A. Conversion of maleic acid to fumaric acid Mass of fumaric acid + watch glass g Mass of watch glass g Mass of fumaric acid g Percentage yield % B. Comparison of properties of the two isomers 1. Water solubility: 2. Melting point of maleic acid: o Melting point of fumaric acid: o 3. acid is more soluble. C C Acid strength Test Maleic acid pH of solution Reaction with Mg Reaction with Na2CO3 4. Reaction with bromine water Maleic acid: Fumaric acid: P.4 Fumaric acid Experiment O07 Investigation of some of the properties of a pair of cis-trans isomers Questions: 1. Assuming that equilibrium concentration was achieved in procedure A, which isomer would you classify as the more stable with respect to transformation of one into the other? 2. Explain the difference in reaction with magnesium and sodium carbonate between maleic acid and fumaric acid. 3. Considering the structures of the two isomers, try to account for the observed differences in solubility and melting point. 4. One of the isomers can lose a molecule of water from each molecule of acid when its two carboxyl groups react to form an anhydride. Which geometrical isomer, cis- or trans-, do you predict it is? P.5