expt tech notes blanks
advertisement

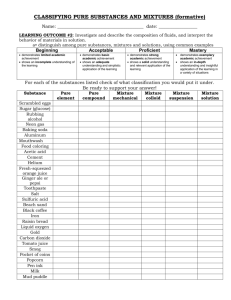
Experimental Techniques Contents: Learning Objectives ................................................................................................................... 2 1. Common Measuring Instruments ........................................................................................... 3 1.1 Measuring Time ........................................................................................................... 3 1.2 Measuring Temperature ............................................................................................... 3 1.3 Measuring Mass ........................................................................................................... 3 1.4 Measuring Volume ....................................................................................................... 4 2. Mixtures & Pure Substances .................................................................................................. 5 2.1 Pure Substances ............................................................................................................ 5 2.2 Mixtures ....................................................................................................................... 5 2.3 Purification ................................................................................................................... 5 3. Methods of Purification/Separation ....................................................................................... 6 3.1 Filtration ....................................................................................................................... 6 3.2 Crystallisation............................................................................................................... 7 3.3 Use of Separating Funnel ............................................................................................. 9 3.4 Sublimation ................................................................................................................ 10 3.5 Simple Distillation...................................................................................................... 11 3.6 Fractional Distillation ................................................................................................. 13 3.7 Paper Chromatography ............................................................................................... 15 4. Criteria of Purity................................................................................................................... 17 4.1 Importance of Purity................................................................................................... 17 4.2 Test for Purity............................................................................................................. 17 1 Learning Objectives At the end of this unit, you should be able to: 1. Name appropriate apparatus for the measurement of time, temperature, mass and volume. 2. Describe the methods of purification by: (i) Filtration (ii) Crystallisation (iii) The use of a suitable solvent (iv) Evaporation (v) The use of separating funnel (vi) Sublimation (vii) Simple distillation (viii) Fractional distillation } } }often used together } 3. Describe paper chromatography and interpret chromatograms. 4. Describe how chromatography techniques can be applied to colourless substances (knowledge of the composition of particular locating agents is not required). 5. Suggest suitable purification techniques, given information about the substances. 6. Design arrangements of apparatus, given information about the substances involved. 7. Explain the importance of purity in substances in everyday life, example in foodstuffs and drugs. 8. Identify substances and test their purity by melting point and boiling point determination, and by paper chromatography. 2 1. Common Measuring Instruments 1.1 Measuring _________ Name: _____________ Units: _____________ 1.2 Measuring ________________ Name: _________________ Units: _________ This is a _________________________________. Electronic thermometers are increasingly being used nowadays, especially in clinics and hospitals. 1.3 Measuring ___________ (i) Name: ___________________________ (ii) Name: __________________________ Units: ___________ Units: ____________ The ________ of a substance is a measure of the amount of ____________ contained in it. 3 1.4 Measuring ____________ Units for the following five apparatus: _______________. (i) Name: __________ (approximate measurement) (iv) Name: _____________ (very accurate) (iii) Name: ____________ (very accurate) meniscus (ii) Name: ____________________ (more accurate) The beaker, measuring cylinder, burette and pipette are usually used for measuring the ________________ of _____________. The pipette measures a __________________ volume of liquid when it is filled to a mark. (iv) Name: ___________________ Syringes of different sizes can be used to measure the _______________ of ___________ or ___________. Small plastic syringes are used to measure small volumes of liquids. Example: doctors use it to give a patient an injection. Larger ones are used to measure volumes of gases. 4 2. Mixtures & Pure Substances 2.1 Pure Substances A _________ substance is a ___________ substance ______________ with anything else. Example: sugar crystals. It has ______________ properties, such as a __________ melting point and boiling point. Example: pure water melts at 0oC and boils at 100oC. 2.2 Mixtures A ______________ contains __________________ substances. Examples: ________________ (________________________), _______ (____________________________________________________), It ______________ have definite properties. It _____________ have fixed melting and boiling points. It melts and boils over ______________ of several degrees of temperature. Examples: Coconut oil used for cooking starts melting at 14oC and completes melting at 22oC. Petrol fuel for motorcars has a boiling point range of 35oC to 75oC. 2.3 Purification Mixtures can easily be separated into pure substances. The process is called ______________________. This is done by physical methods. No chemical reactions are needed. Several of these methods will be discussed in the next Section. 5 3. Methods of Purification/Separation 3.1 Filtration Purpose Filtration is a technique used to separate a __________ from a ___________. Example: sand from water. Procedure & Setup of Apparatus – Example: Separation of sand from a mixture of salt, sand & water Set up the apparatus shown below. Pour the mixture through __________________ in a ____________________. Collect the filtrate in a beaker. Notes The liquid passing through the filter paper is called the ________________. The solid collected on the filter paper is called the ________________. How does it Work? The filter paper has tiny holes through which particles of the liquid are able to pass. The particles of the solid, being large, cannot pass through these holes and are trapped by the filter paper. Practical Application The removal of solid impurities by filtration is one of the steps in making ____________________________. 6 3.2 Crystallisation Some background knowledge: A solid _____________ in a liquid to form a ________________. The solid that dissolves is called a __________ and the liquid is called a _____________. A _______________ solution contains the _____________ amount of solute that can be dissolved at a particular __________________. Purpose of Crystallisation To separate the ____________ (usually a salt) from the _______________ in a solution. Example: copper (II) sulphate crystals from aqueous copper (II) sulphate solution. Crystallisation is often used together with: Dissolution: _______________ a solute in a solvent. Evaporation: a solution is _______________ to dry off the solvent. If all the solvent is driven off, leaving a solid residue, the process is called evaporation to dryness. A water bath is used if the solvent is flammable or to prevent damage to the solute. Filtration Procedure & Setup of Apparatus – Example: Making pure salt from impure salt The impure salt contains salt (soluble in water) and impurities (insoluble in water). Step 1: Dissolution Place the given impure salt in a beaker and add about 50 cm3 of _______________ (this is a suitable solvent). _________ while _____________ the mixture until the salt appears to have dissolved. 7 Step 2: Filtration Filter the hot mixture by pouring it through ___________________ in a _____________________. The solid impurities remain on the filter paper (residue). Collect the salt solution (filtrate) in a beaker. Step 3: Evaporation _________ the filtrate in an _______________________________ to evaporate most of the water. Step 4: Cooling Allow the _______________________ solution to cool. Crystals of the pure salt will form. Pour off the cold solution to obtain the crystals. ________ the crystals between filter paper. 8 After crystallisation, The crystals can be weighed and the percentage purity of the impure salt can be calculated using: Mass of salt obtained Purity = x 100% Initial mass of impure salt Practical Applications of Crystallisation (1) Manufacture of sugar. (2) Manufacture of silicon crystals. 3.3 Use of Separating Funnel Purpose To separate two ___________________________. Example: water and cooking oil. Procedure & Setup of Apparatus – Example: Separation of water & cooking oil Pour the mixture into a ________________________ and leave it standing for some time. When the layers of liquids have separated, open the tap to collect each layer in separate beakers. How does it Work? ___________________________________________________________________________ ___________________________________________________________________________ 9 3.4 Sublimation Recall: Sublimation is the conversion of a _____________ into a _________________ without the solid first melting. Purpose of Sublimation To separate __________________________________ from _____________________ ______________________. Examples of solids that sublime are iodine and ammonium chloride. Procedure & Setup of Apparatus - Example: Separation of a mixture of ammonium chloride and sodium chloride Set up the apparatus shown below. _________ the mixture __________________. The ammonium chloride _________________ and ___________________ on the cooler sides of the filter funnel. Practical Application Sublimation is used industrially in the purification of solids that sublime. 10 3.5 Simple Distillation Purpose To separate a pure ______________ from a ___________________ of a solute. It purifies the liquid. Example: distillation of sea water to obtain pure water. Procedure & Setup of Apparatus - Example: Obtaining water from sea water Set up the apparatus as shown below: __________ the sea water in the flask. __________ the steam produced using a _____________________. Collect the pure liquid water obtained. The salt remains in the flask. 11 How does it Work? In distillation, the liquid (the solvent) is changed into a gas by __________________. This gas is pure as other substances are left behind. So in the sea water distillation example: when the sea water boils, pure gaseous water (steam) is produced. The gas is then _____________. It condenses into a pure liquid which is called the __________________. So in the sea water distillation example: steam condenses into liquid water. Sea water is a solution of solid salts in water. The salts, like other solids dissolved in water, cannot be distilled and remain in the flask. Notes The thermometer should be placed _____________ the liquid level in the flask, and next to the opening into the condenser. In the sea water distillation example, the thermometer shows a temperature of 100oC during the distillation. This is the ____________________________ of water. 12 3.6 Fractional Distillation Purpose To separate a mixture of ___________________________. These liquids are completely soluble in one another. They mix to form one liquid. Example: fractional distillation of a mixture of ethanol and water. Procedure & Setup of Apparatus – Example: Obtaining ethanol from a mixture of ethanol & water A ___________ _____________ is used. Boiling points: Ethanol: 78oC Water: 100oC Set up the apparatus shown on the right. Boil the mixture of ethanol and water in the flask. Collect the liquid that is distilled out at 78oC (boiling point of ethanol). 13 How does it Work? A fractionating column separates liquids in order of their _____________________. The liquid with the ____________ boiling point is distilled __________. The liquid with the ____________ boiling point is distilled __________. In the example, the mixture to be separated contains ethanol and water. Since ethanol has a lower boiling point (78oC) than water (100oC), it boils ___________. The ethanol ______________ reaches the top of the fractionating column and is then ___________ as it passes through the _________________. It condenses into ____________ ethanol and is collected. Notes The thermometer should be placed ___________ the liquid level in the flask, and next to the opening into the condenser. While the first liquid (in this case, ethanol) is being distilled, the thermometer shows the boiling point of this liquid (in this case, 78oC). The liquids with higher boiling points remain in the flask (in this case, only water). When almost all of the ethanol has distilled, the water distils. When this happens, the thermometer shows a temperature of 100oC. In the example, can ethanol and water be completely separated? That is, pure ethanol and pure water will be obtained? ________________________________________________________________________ ________________________________________________________________________ Practical Applications (1) Fractional distillation is used in the petroleum industry to separate __________________ into useful fractions, such as petrol and diesel. In this case the mixture to be separated contains a number of substances with different boiling points. (2) Ethanol is produced in the industry by the fermentation of cane sugar (wine making). The product of fermentation is a mixture of ethanol and water. Ethanol is obtained from this mixture by fractional distillation. 14 3.7 Paper Chromatography Purpose Chromatography is a method of separating and identifying mixtures. There are several types of chromatography, one of which is ________________________. Example: separation of the dyes in black ink. Procedure & Setup of Apparatus - Example: Separation & identification of dyes in black ink Draw a pencil line on a piece of chromatography paper. Place a drop of black ink on the pencil line. The black ink is suspected to be a mixture of red, blue, green and orange dyes. So place drops of these dyes on the pencil line too. Roll the paper into a cylinder and secure it. Place it into a beaker containing a suitable solvent for the ink and dyes. (The chromatography paper can also be suspended from a glass rod, such that the end of the filter paper is dipped into the solvent.) The solvent travels up the paper. The dyes become separated. When the solvent reaches the top of the paper, take it out of the beaker and leave it to dry. 15 How does it Work? In paper chromatography, a suitable _______________ for the mixture to be separated has to be selected. In the example, the dyes in black ink are to be separated and identified. As the solvent travels up the paper, the dyes on the pencil line dissolve in the solvent. The ______ soluble the dyes are in the solvent, the ___________ they move up the paper. Hence the dyes move up the paper at _____________________ and become __________. A chromatogram is produced: Identical dyes travel the _______________________ up the paper. Unknown dyes in the black ink can be identified by comparing the chromatogram obtained for the black ink, with chromatograms of known dyes. Results of the experiment show that: - the black ink is made up of __________ different dyes; - three of the dyes are the known _________, _________ and __________ dyes; - there is no _____________ dye in the black ink; - there is one dye that is different from the four known dyes. Main Advantage of Chromatography It can be used to identify very tiny amounts of substances (10-12 gram or less). Practical Applications (1) Chromatography can be used to separate and identify complicated substances such as __________ and __________________ in food. Government laboratories use chromatography to check that only approved dyes are used in food. (2) Analysing the ____________________ of athletes to check if they are using illegal drugs. 16 4. Criteria of Purity 4.1 Importance of Purity The separation of mixtures into pure substances is important. Chemists need pure substances to study their properties. Pure substances are used in industry to make useful products such as food and drugs. What is the danger if there are impurities in the food and drugs? ________________________________________________________________________ The ‘chips’ used in calculators, computers, watches and compact disc players are made of silicon. Very pure silicon crystals are required. 4.2 Test for Purity (i) Melting Point Determination Impurities ____________ the melting point. The greater the percentage of impurity, the ______________ the melting point. We can find out if a substance is pure by measuring its melting point, and then compare the measured value to the true melting point recorded in books. This method is usually applied to _____________. Another way: check if the substance melts over a _____________ of temperature. Why? ______________________________ ______________________________ ______________________________ Note: The thermometer bulb should be placed _________________ the Setup of Apparatus capillary tube. 17 (ii) Boiling Point Determination Impurities ____________ the boiling point. We can find out if a substance is pure by distilling it. If the substance is pure, all of it distils at the _________ temperature, which is the _________________________. Otherwise, it will distil over a ___________ of temperatures. This method is usually applied to ____________. Note: The thermometer should be placed ___________ the liquid level in the flask. the substance to be checked for purity Setup of Apparatus (iii) Chromatography This method is used for complicated substances and those that cannot be melted or distilled. It is a very sensitive test and is able to detect very _____________________ of impurity. As shown by the diagram below, a pure substance gives ______ spot on a chromatogram. A mixture gives _____________________ on a chromatogram. Chromatography can also be used to separate and identify colourless substances. The chromatogram is sprayed with a ___________________________to show where the substances are on the paper. The locating agent is a chemical that reacts with the substances to produce a ________________ product. 18